The use of boranes in a promising experimental approach to cancer treatment is one of the most challenging current targets of medicinal chemistry. Fmoc-8-amino-3,6-dioxaoctanoic acid web
Amine-carboxyboranes
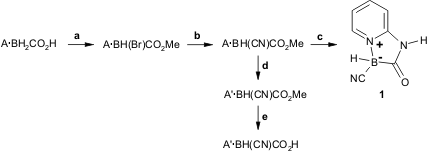
Amine-carboxyboranes (A•BH2COOH) are boron analogues of protonated α-aminoacids. These compounds, their derivatives (A•BH2COR), and the precursor amine-cyanoboranes present significant biological activity (J. Pharm. Sci. 1979, 68, 685; J. Pharm. Sci. 1980, 69, 1025.). Their use as boron carriers to tumour cells for boron neutron capture therapy (BNCT) has been suggested (Radiat. Res. 1197020-22-6 Price 2001, 156, 118.Link; Collect. Czech. Chem. Commun. 2002, 67,1095.DOI: 10.1135/cccc20021095). PMID:24580853 Bényei et al. published the first report of an amine-cyanocarboxyborane 1 that contains a chiral boron atom (J. Organometallic Chem. 2004, 689, 3567–3581.DOI: 10.1016/j.jorganchem.2004.06.066).
a) N-bromosuccinimide, MeOH, rt, 30 min, 87%; b) 1.5 eq. [Bu4N]CN, MeCN, 71-84 %; c)3 eq. 2-aminopyridine, MeCN, reflux 22 h, water, reflux, 1 h, 39%; d) 2–3 eq. of A’, MeCN, reflux, 2–14 h, 44-79%; e) 0.45 eq. HCl in 0.05-0.2M HCl in water or water–acetone mixture, 55-100 ºC, 0.5-2.5 h, 38-88%. A or A’= amine; aromatic, aliphatic, cyclic or linear.
Carboranes
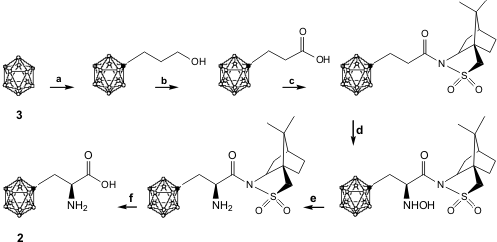
In the last 10 years, high boron content carboranes (C2B10H12carbon-boron clusters arranged in an icosahedral geometry in which the carbon and boron atoms are hexacoordinated) were studied for use in BNCT for cancer (Coordination Chemistry Reviews 2002, 232, 173.DOI: 10.1016/S0010-8545(02)00087-5). Carboranes, themselves, are remarkably stable (to oxidising agents, alcohols and strong acids, and up to 400 ºC); however, they are biologically inactive since they are too hydrophobic to engage in ionic or hydrogen bonding being however biologically inactive since they are too hydrophobic to make ionic or hydrogen bonding (Chem. Rev. 1992, 92, 209.) Their use as hydrophobic pharmacophores in biologically active molecules has been reported and reviewed (Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1203.). The Sjöberg group has recently reported an enantioselective synthesis of m-carboranylalanine 2 (19% overall yield, 98% ee) starting from m-carborane 3 (Tetrahedron,2005, 61, 1181.DOI: 10.1016/j.tet.2004.11.045).
BH = white dots, CH = grey dots, C = black dots
a) 1. BuLi, TBDMS-Cl; 2. BuLi, oxetane; 3. (n-C4H9)4NF;b) CrO3, H2SO4/acetone. c), Oppolzer’s sultam, DCC, DMAP, CH2Cl2; d) NaN(TMS)2, 1-chloro-1-nitrosocyclohexane,THF; e) 1. Zn-dust, HOAc/HCl, 2. NaHCO3;f) 1. LiOH, 2. HCl.
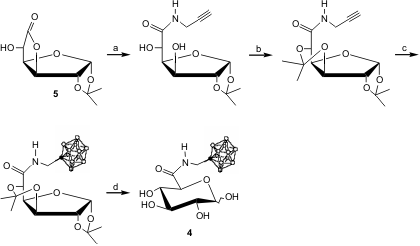
Panza and coworkers have reported the synthesis of carborane-containing carbohydrates 4 (glucuronic acid derivatives bearing an o-carborane), starting from the commercially available lactone 5 (49% overall yield), which are potential BNCT compounds. The sugar moiety increases the water solubility of the boron cluster and may also enable a targeting effect on tumour cells (Tetrahedron Asymmetry, 2005, 16, 39.DOI: 10.1016/j.tetasy.2004.11.059).
a) 2-propynyl amine (1.3 eq.), rt, overnight, THF; 95% b)2,2-dimethoxypropane (7.1 eq.), camphorsulphonic acid (0.05 eq.), acetone, rt, overnight, 85%; c) 1. decaborane (1.2 eq.), toluene/acetonitrile, reflux, 1h; 1. compound 3, reflux, 18h, 61%; d) 90% aq. CF3CO2H, 10 min., quantitative.