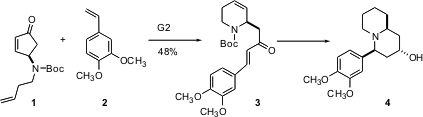
Practitioners of total synthesis have been pushing the limits of Grubbs metathesis. Siegfried Blechert of the Technisches Universität, Berlin, envisioned (Tetrahedron 2004, 60, 9629.DOI: 10.1016/j.tet.2004.06.145)that Grubbs metathesis of 1 could open the cyclopentene, to give a new Ru alkylidene that could condense with a styrene such as 2. Price of (R)-N-Fmoc-2-(7-octenyl)Alanine 39692-67-6 site In practice, this transformation worked well, yielding 3. PMID:24118276 Deprotection, intramolecularMichael addition and reduction then gave (-)-lasubine II 4.
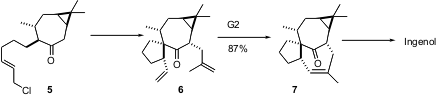
The twisted inside-outside skeleton of ingenol has long challenged organic chemists. Hideo Kigoshi of the University of Tsukuba conceived (J. Org. Chem. 2004, 69, 7802.DOI: 10.1021/jo048833l)that metathesis of 6 could lead to 7, leading directly into an established ingenol end game. The diene 6 was readily prepared by intramolecular alkylation of 5, with bond formation occurring away from the adjacent methyl group. As expected. Alkylation with methallyl iodide then proceeded exo on the cis-fused bicyclic skeleton, to give the desired 6. The key question was whether or not the two alkenes of 6 could find each other in the metathesis reaction. In fact, this proceeded smoothly. Alkene 7 likely does not react with the Grubbs catalyst, so once the ring forms, it cannot reverse.
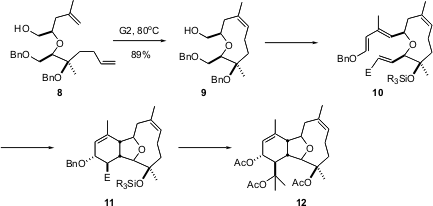
There are times that it is important to run the Grubbs reaction under equilibrating conditions. In the course of a synthesis of ophirin B (12), Michael Crimmins of the University of North Carolina observed (J. Am. Chem. Soc. 2004, 126, 10264.DOI: 10.1021/ja046574b)that metathesis of 8 under the usual conditions gave mainly the undesired cyclic dimer. At elevated temperature, the dimer re-entered the equilibrium, leading to the desired 9 as the major (15:1) product. The oxonene ring system of 10 then directed the intramolecular Diels-Alder cycloaddition, leading to 12.