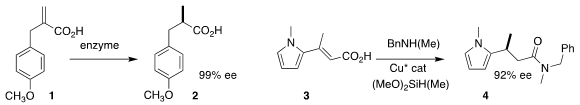
Chihui An and Megan H. Shaw of Merck Process achieved high ee in the
reduction of the α-methylene acid 1 to the α-methyl acid 2
(Org. Lett. 2020, 22, 8320.
DOI: 10.1021/acs.orglett.0c02959).
Stephen L. Buchwald of MIT devised the enantioselective
conjugate
reduction of the unsaturated acid 3 to the amide 4
(Org. Lett. 2020, 22, 5666.
DOI: 10.1021/acs.orglett.0c02064). Bromo-PEG2-C2-azide In stock
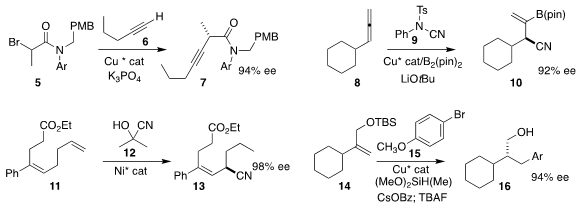
Guozhu Zhang of the Shanghai Institute of Organic Chemistry assembled the α-branched
alkyne
7 by coupling the α-bromo amide 5 with the terminal alkyne 6
(Angew. PMID:23891445 Chem. Int. Ed. 2020, 59, 13998.
DOI: 10.1002/anie.202000860).
Liang Zhang and Zhaomin Hou of RIKEN and Gen Luo of Anhui University added the
N-sulfonylcyanamide 9 to the allene 8, to give the nitrile 10
(ACS Catal. 2020, 10, 11685.
DOI: 10.1021/acscatal.0c03018).
Xianjie Fang of Shanghai Jiao Tong University used acetone cyanohydrin
(12) and a Ni catalyst to accomplish the migratory hydrocyanation
of 11, leading to the allylic nitrile 13
(Angew. Chem. 3-(Hydroxymethyl)oxetane-3-carbonitrile Chemscene Int. Ed. 2020, 59, 21436.
DOI: 10.1002/anie.202008854).
Professor Buchwald and Zhaohong Lu, also of MIT, devised the reductive Heck
type addition of the bromoarene 15 to the allylic ether 14,
to give the α-branched alcohol 16
(Angew. Chem. Int. Ed. 2020, 59, 16128.
DOI: 10.1002/anie.202004414).
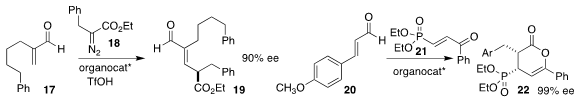
Sanzhong Luo of Tsinghua University used a diamine to mediate the coupling of
the α-methylene aldehyde 17 with the α-diazo ester 18,
to prepare the net C-H insertion product 19
(ACS Catal. 2020, 10, 10989.
DOI: 10.1021/acscatal.0c02405).
Bhoopendra Tiwari of the Centre of Biomedical Research used an organocarbene catalyst to construct
the δ-lactone 22 by the combination of the unsaturated aldehyde 20 with the
phosphoryl enone 21
(Chem. Commun. 2020, 56, 7155.
DOI: 10.1039/D0CC03204B).
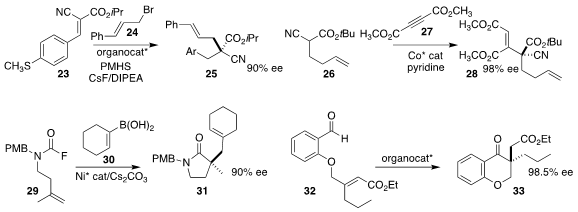
Richmond Lee of the University of Wollongong, Haihua Lu of Westlake University
and Choon-Hong Tan of Nanyang Technological University used a bis-guanidinium salt
to direct the reductive alkylation of the unsaturated cyano ester 23 with
cinnamyl bromide 24, leading to 25
(J. Am. Chem. Soc. 2020, 142, 19065.
DOI: 10.1021/jacs.0c00183).
Quang H. Luu and John A. Gladysz of Texas A&M developed a Co catalyst that directed the addition
of the cyano ester 26 to the acetylenic diester 27, to give 28
(Chem. Eur. J. 2020, 26, 10230.
DOI: 10.1002/chem.202001639).
Yu-Xin Luan and Mengchun Ye of Nankai University assembled the
γ-lactam
31 by cyclizing the carbamoyl fluoride 29 with a Ni catalyst,
then coupling the intermediate from that cyclization with the boronic acid 30
(J. Am. Chem. Soc. 2020, 142, 19844.
DOI: 10.1021/jacs.0c09949).
Zbigniew Rafiński of the Nicolaus Copernicus University in Torun used an
NHC catalyst to cyclize the allylic ether 32 to the benzo-fuzed pyranone 33 in high ee
(Adv. Synth. Catal. 2020, 362, 3830.
DOI: 10.1002/adsc.202000550).
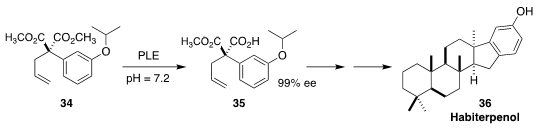
Habiterpenol (36), isolated from a culture of Phytohabitans sp. 3787-5, is an
otherwise nontoxic specific inhibitor of the G2 DNA damage repair checkpoint.
Tohru Nagamitsu of Kitasato University established the absolute configuration of
one of the cyclic quaternary centers of 36 by the selective enzymatic
hydrolysis of the diester 34 to the monoester 35
(Org. Lett. 2020, 22, 5131.
DOI: 10.1021/acs.orglett.0c01736).