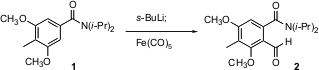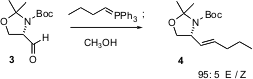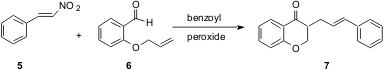Carbon-carbon bond formation is fundamental to all of organic chemistry. 5-Fluoro-2-hydroxybenzonitrile Formula The emphasis again this week is on practical, scalable methods.
Formylation of organometallics is usually carried out with dimethylformamide (DMF). 1175052-07-9 Chemscene T. Ross Kelly of Boston College found (J. Org. PMID:24518703 Chem. 2004, 69, 2191. DOI: 10.1021/jo0356210)that although o-lithiation of 1 proceeded smoothly, formylation with DMF and with methyl formate failed. The inexpensive Fe(CO)5, however, worked smoothly.
The Wittig reaction can be controlled to give a high percentage of the cis alkene. Young Gyu Kim of Seoul National University has shown (Tetrahedron Lett. 2004, 45, 3925.DOI: 10.1016/j.tetlet.2004.03.101)that addition of an excess of methanol to the -78°C Wittig reaction of 3 led to the complementary trans alkene 4.
Another way to construct alkenes is by the addition of carbon radicals to nitrostyrenes such as 5. Ching-Fa Yao of National Taiwan Normal University in Taipei has reported (J. Org. Chem. 2004, 69, 3961.DOI: 10.1021/jo049763l)an extension of this work, generating the acyl radical from the aldehyde 6, cyclizing it to generate a new radical, then trapping that radical with 5 to give 7. This article includes an overview of the several ways of adding radicals to 5.
Telescoping of reaction steps, where feasible, can significantly improve the overall efficiency of a target-directed sequence. Zoapatanol 10 features a delicate combination of organic functional groups, in particular the α,β-unsaturated ketone. In the last step of a total synthesis of 10 (Org. Lett. 2004, 6, 2149. DOI: 10.1021/ol049433n), Janine Cossy of ESPCI, Paris, describes the addition of prenyl lithium to the Weinreb amide 8. The intermediate 9 was stable to dissolving metal reduction, where the ketone 10 would not have been. Work up then gave zoapatanol 10. In the preceding paper (Org. Lett. 2004, 6, 2145.DOI: 10.1021/ol049434f), the authors describe in detail the development of this method. Note that in natural zoapatanol 10, the stereogenic center adjacent to the ketone is a 1:1 epimeric mixture.