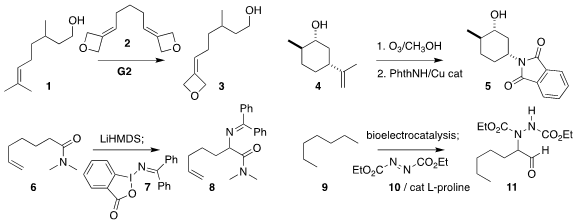
Imre Pápai and Tibor Soós of the Institute of Organic Chemistry, Budapest
prepared the oxetane 3 by the relay
ring-closing methathesis of the alkene
1 with the diene 2
(Angew. 126689-04-1 Chemscene Chem. Int. Ed. Propargyl-PEG1-NHS ester web 2023, 62, e202216879.
DOI: 10.1002/anie.202216879).
Ohyun Kwon of UCLA ozonized the alkene
4 to an intermediate that under Cu catalysis could be
coupled with phthalimide, leading to the protected
amino alcohol 5
(Science 2023, 381, 877.
DOI: 10.1126/science.adi4758).
Kensuke Kiyokawa and Satoshi Minakata of Osaka University
assembled the α-amino amide 8 by coupling the amide 6 with the hypervalent
iodine reagent 7
(Chem. Eur. J. PMID:24238415 2023, 29, e202203722.
DOI: 10.1002/chem.202203722).
Hui Chen and Shelley D. Minteer of the University of Utah devised the three-stage oxidation of heptane (9) followed by coupling with
10, leading to the α-amino aldehyde 11
(ACS Catal. 2023, 13, 563.
DOI: 10.1021/acscatal.2c04003).
Jieping Zhu of the Ecole Polytechnique Fédéral de Lausanne effected the
oxidative ring expansion of the alkene 12 to the
cycloheptanone 13
(Science 2023, 379, 1363.
DOI: 10.1126/science.adg3182).
Mu-Hyun Baik of KAIST, Djamaladdin G. Musaev of Emory
University and Richmond Sarpong of the University of California, Berkeley
prepared the aldehyde 15 by the oxidative cleavage of the amide 14
(J. Am. Chem. Soc. 2023, 145, 11245.
DOI: 10.1021/jacs.3c01318).
E. J. Corey of Harvard University reported the use of nitrosyl triflate to selectively convert caryophyllene
(16) to the keto aldehyde 17
(Org. Lett. 2023, 25, 1872.
DOI: 10.1021/acs.orglett.3c00353).
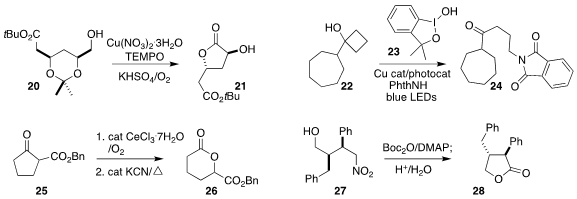
Liangzhen Hu and Yan Xiong of Chongqing University prepared the
α-acetoxy ketone 19 by the oxidation of the propiolic acid 18
(Org. Biomol. Chem. 2023, 21, 1457.
DOI: 10.1039/D2OB02281H).
Hui Qian of Fudan University and Shengming Ma of the Shanghai Institute of
Organic Chemistry achieved the selective oxidation of the
acetonide 20 to the
lactone 21
(Chem. Commun. 2023, 59, 5281.
DOI: 10.1039/D3CC00963G).
Xiao-song Xue and Yiyun Chen, also of
the Shanghai Institute of Organic Chemistry, used the dimethyl benziodoxole 23
to activate both the cyclobutanol 22 and phthalimide, leading to the protected
amine 24
(ACS Catal. 2023, 13, 3749.
DOI: 10.1021/acscatal.3c00230).
In an interesting alternative to
Baeyer-Villiger
oxidation, Jens Christoffers of the Carl von Ossietzky
Universität Oldenburg α-hydroxylated the β-keto ester
25, then used cyanide to
rearrange the resulting alcohol to the lactone 26
(Eur. J. Org. Chem. 2023, 26, e202201391.
DOI: 10.1002/ejoc.202201391).
Hongya Li and Yu Yuan of the University of Central Florida oxidized
the nitro alcohol 27 to the lactone 28
(Org. Lett. 2023, 25, 31.
DOI: 10.1021/acs.orglett.2c03727).
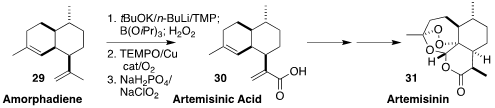
Artemisinic acid (30) is the key precursor to the important anti-malarial
artemisinin (31). Using an optimized version of tetramethylpiperidide
stoichiometric deprotonation, Doug E. Frantz of the University of Texas at San
Antonio effected the direct oxidation of the readily-prepared amorphadiene (29) to
the crystalline 30
(Org. Lett. 2023, 25, 277.
DOI: 10.1021/acs.orglett.2c04145).