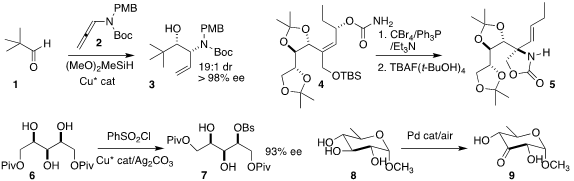
Joshua D. Sieber of Virginia Commonwealth University used a Cu catalyst to
mediate the assembly of the protected
amino alcohol 3 by the addition of the
allenamide 2 to the aldehyde 1
(Org. Ethyl 4-aminopyrimidine-5-carboxylate Chemscene Lett. 2023, 25, 4644,
DOI: 10.1021/acs.orglett.3c01459;
4730, DOI: 10.1021/acs.orglett.3c01698)
Yoshiyasu Ichikawa of Kochi University used an allyl cyanate to isocyanate rearrangement
to convert the carbamate 4 to the
oxazolidinone 5
(Heterocycles 2023, 106, 649.
DOI: 10.3987/COM-23-14817).
Xin Hong of Zhejiang University and Qiang-Shuai Gu and Xin-Luan Liu of the
Southern University of Science and Technology effected enantioselective
derivitization of the prochiral triol 6, leading to the sulfonate 7
(Nature Chem. 2023, 15, 395.
DOI: 10.1038/s41557-022-01102-z).
Robert M. Waymouth of Stanford University selectively
oxidized the sugar derivative
8 to the ketone 9
(J. Am. PMID:24078122 Chem. Soc. 6-Bromo-3-chloroisoquinoline In stock 2023, 145, 2282.
DOI: 10.1021/jacs.2c10667).
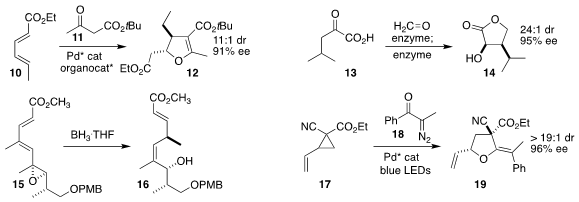
Zhi-Tao He of the Shanghai Institute of Organic Chemistry used a synergistic
combination of a Pd catalyst and a Cinchona-derived amino alcohol to assemble
the dihydrofuran
12 by coupling ethyl sorbate (10) with the β-keto ester 11
(Angew. Chem. Int. Ed. 2023, 62, e202215568.
DOI: 10.1002/anie.202215568).
Pere Clapés of the Institute for Advanced
Chemistry of Catalonia used an enzyme-mediated formaldehyde
aldol reaction followed by an
enzymatic reduction to convert the α-keto acid 13 into the
lactone 14
(ACS Catal. 2023, 13, 5348.
DOI: 10.1021/acscatal.3c00367).
Shinji Nagumo of Kogakuin University observed high diastereocontrol in the reduction
of the epoxy diene 15 to the allylic alcohol 16
(Chem. Asian J. 2022, 17, e202200650.
DOI: 10.1002/asia.202200650).
Li Rao and Liang-Qiu Lu of Central
China Normal University prepared the
tetrahydrofuran
19 by irradiating the diazo
ketone 18 in the presence of the activated cyclopropane 17
(Angew. Chem. Int. Ed. 2023, 62, e202212444.
DOI: 10.1002/anie.202212444).
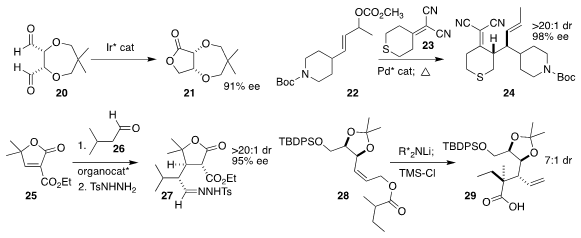
Takeyuki Suzuki of Osaka University developed the enantioselective
Tishchenko
transformation of the prochiral dialdehyde 20 to the lactone 21
(Tetrahedron 2023, 133, 133287.
DOI: 10.1016/j.tet.2023.133287).
Alexander J. Grenning of the University of Florida
constructed the diene 24 by coupling the dinitrile 23 with the allylic carbonate
22, followed by Cope rearrangement
(Chem. Sci. 2023, 14, 2755.
DOI: 10.1039/D2SC07021A).
Jingxiang Pang, Aiqin Liu and Zhushuang Bai of the Shandong First Medical University used the
Hayashi-Jørgensen catalyst to mediate the
conjugate addition of the aldehyde 26
to the unsaturated lactone 25, then isolated the product as the tosylhydrazone
27
(Org. Chem. Front. 2023, 10, 1527.
DOI: 10.1039/D2QO01891H).
Armen Zakarian of the University of
California, Santa Barbara showed that using a lithiated diamine as base, the
Ireland-Claisen rearrangement of the allylic ester
28 delivered the rearranged
acid 29 with high diastereoselectivity
(J. Org. Chem. 2023, 88, 7560.
DOI: 10.1021/acs.joc.3c00535).
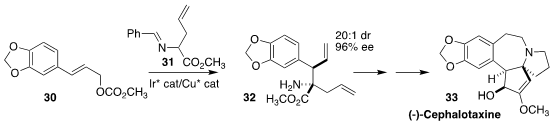
Cephalotaxine (33) was isolated from the Japanese plum yew Cephalotaxus
drupacea. The esters of 33 show important anti-cancer activity. Building on the
results of Wang and of Zhang
(![]() 2019, February 25.),
2019, February 25.),
Kun Wei and Yu-Rong Yang of the Kunming Institute of Botany coupled the protected amino ester 31 with the
allylic carbonate 30, leading to 31, having two of the three stereogenic centers of
33 (Org. Chem. Front. 2023, 10, 2532.
DOI: 10.1039/D2OB00486K).
We note that the journal Heterocycles suspended publication at the end of 2023.
We appreciate the dedication of the editors in keeping this journal going for so
many years.