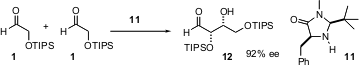Many of the five- and six-carbon sugars, although well known, are rare, and
too expensive to be used as chiral pool starting materials. David MacMillan of
Caltech in an elegant series of papers (Angew. Chem. Int. Ed. 2004,
43, 2152,
DOI: 10.1002/anie.200453716;
Science 2004, 305, 1752,
DOI: 10.1126/science.1101710;
Angew. Chem. Int. PMID:28038441 Ed. tert-Butyl 7-bromoheptanoate Price 1339559-21-5 structure 2004, 43, 6722,
DOI: 10.1002/anie.200461851.)
has demonstrated a two-step route not just to protected six-carbon carbohydrates,
but also to alkyl, thio and amino derivatives of those carbohydrates.
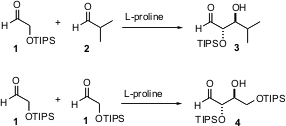
The key observation was that L-proline would catalyze the addition of α-hetero
aldehydes to α-branched aldehydes such as 2 to give the aldol product
3 with high enantio- and diastereocontrol. Even more exciting, in the
absence of other acceptors the α-hetero aldehydes dimerize with high
relative and absolute stereocontrol. Both alkoxy and silyloxy aldehydes worked
efficiently.
The second step in the hexose synthesis was the aldol condensation of 4
with another α-hetero aldehyde derivative 5. By tuning the Lewis acid and
the solvent, three of the four possible diastereomeric products could be
selectively prepared. α-Amino and α-thio aldehydes worked well also, leading to
9 and
10 respectively.
Proline catalysis leads to the anti products 3 and 4. Use
of the designed imidazolidinone catalyst 11 leads to the complementary
syn product
12.