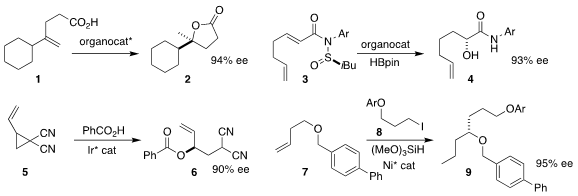
Benjamin List of the Max-Planck-Institut für Kohlenforschung used a confined
imidodiphosphorimidate to catalyze the cyclization of the unsaturated acid 1 to
the lactone 2
(J. Am. Chem. Soc. 2023, 145, 8788.
DOI: 10.1021/jacs.3c01404).
Nicolai Cramer of the Ecole Polytechnique Fédérale de Lausanne catalyzed
the rearrangement of the N-sulfinyl acrylamide 3 to the
α-hydroxy amide
4 with a 1,3,2-diazaphospholene
(Angew. Chem. Int. Ed. PMID:24220671 2023, 62, e202301076.
DOI: 10.1002/anie.202301076).
Chong-Dao Lu of Yunnan University described a related route to
α-amino amides
(Org. Price of Fmoc-Phe(4-F)-OH Lett. 2023, 25, 4156.
DOI: 10.1021/acs.orglett.3c01448).
Nilanjana Majumdar of CSIR-Central Drug Research Institute assembled the ester
6 by coupling the activated cyclopropane 5 with benzoic acid
(ACS Catal. 2023, 13, 6261.
DOI: 10.1021/acscatal.3c00959).
Xi Lu and Yao Fu of the University of Science and Technology of China
developed conditions for isomerizing the alkene of 7 into conjugation with the
ether oxygen, then coupling the resulting enol ether with the iodide 8 to give 9
(J. Am. Chem. Soc. 2023, 145, 10411.
DOI: 10.1021/jacs.3c02950). 622867-53-2 uses
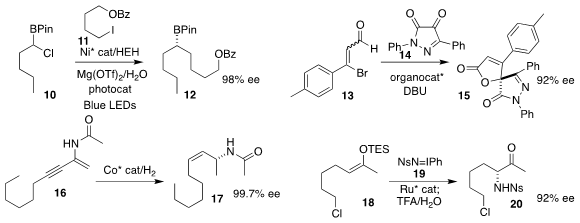
Tao Xu of Tongji University used a Ni catalyst to couple the iodide 11 with
the racemic α-chloroboronate 10, leading to the secondary
organoboronate
12 in high ee
(J. Am. Chem. Soc. 2023, 145, 2081.
DOI: 10.1021/jacs.2c13220).
Alicia Maestro and José M. Andrés of the
Universidad de Valladolid assembled the lactone 15
by NHC-catalyzed [3 + 2]-asymmetric annulation of the β-bromoenal 13 with the 1H-pyrazolo-4,5-dione
14
(J. Org. Chem. 2023, 88, 6890.
DOI: 10.1021/acs.joc.3c00188).
Zhenfeng Zhang and Wanbin Zhang of Shanghai Jiao Tong University hydrogenated
the enamide 16 to the Z-allylic amide 17
(Angew. Chem. Int. Ed. 2023, 62, e202217871.
DOI: 10.1002/anie.202217871).
Shigeki Matsunaga of Hokkaido University prepared the sulfonamide 20 by
aminating the silyl enol ether 18 with the nitrene precursor 19
(Org. Lett. 2023, 25, 3234.
DOI: 10.1021/acs.orglett.3c00940).
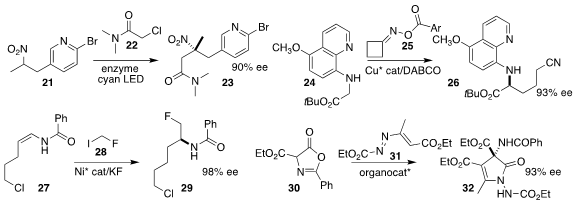
Todd K. Hyster of Cornell University used an enzymatic photoredox catalyst to
mediate the coupling of the nitroalkane 21 with the α-chloro amide
22, leading to the nitro amide 23
(J. Am. Chem. Soc. 2023, 145, 787.
DOI: 10.1021/jacs.2c12197).
Chao Wang and Zhaoqing Xu of Lanzhou University prepared the nitrile 26 by
coupling the cyclobutanone oxime ester 25 with the glycinate 24
(Nature Commun. 2023, 14, 3295.
DOI: 10.1038/s41467-023-38871-1).
Guoqin Xia of the Shanghai Institute of Materia Medica, Huicai Huang of the Guangzhou
University of Chinese Medicine and Zhaodong Li of the Shanghai Institute of
Organic Chemistry prepared the amide 29 by adding the iodide 28 to the enamide
27
(Org. Lett. 2023, 25, 2218.
DOI: 10.1021/acs.orglett.3c00357).
Lihua Huang and Guang-Jian Mei of Zhengzhou University used a chiral phosphoric
acid to construct the
4-pyrrolin-2-one
32 by adding the azlactone 30 to the azoalkene 31
(Chem. Commun. 2023, 59, 5902.
DOI: 10.1039/D3CC01194A).
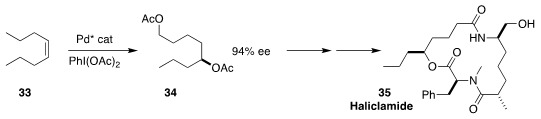
Haliclamide (35), isolated from a Vanuatu marine sponge, Haliclona sp.,
exhibited in vitro antitumor activity against human bronchopulmonary
non-small-cell-lung-carcinoma lines. Zhenyang Lin of the Hong Kong University of
Science and Technology and Guosheng Liu of the Shanghai Institute of Organic
Chemistry developed the enantioselective conversion of (Z)-4-octene (33) to the diacetate
34, a direct precursor to 35
(Nature Chem. 2023, 15, 862.
DOI: 10.1038/s41557-023-01192-3).