Cyclopamine (4), a teratogenic alkaloid isolated from the California
corn lily Veratrum californicum, has been shown to disrupt the hedgehog
signalling pathway. Phil S. Baran of Scripps/La Jolla envisioned a convergent
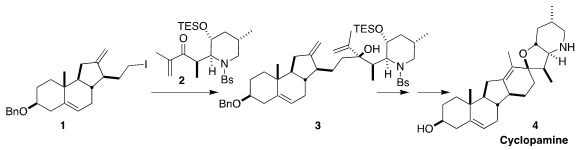
route to 4, assembling the triene 3 by the coupling of 1 and 2,
with the skeleton to be completed by
ring-closing
metathesis (J. Am. Chem. Soc. PMID:23554582 6-Oxa-1-azaspiro[3.3]heptane hemioxalate In stock 2023, 145, 21760.
DOI: 10.1021/jacs.3c09085).
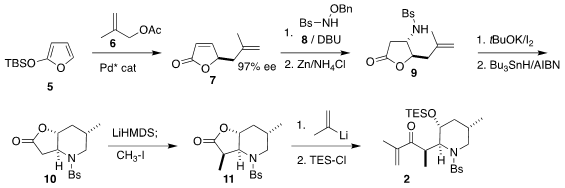
Following the recent precedent of Arseniyadis, the preparation of 2 began
with the Pd-catalyzed addition of methyallyl acetate 6 to the silyloxyfuran
5 to give 7. 6-Bromopyrazin-2-amine site Conjugate addition of the hydroxylamine derivative 8 slightly degraded
the enantiomeric excess, but this could be restored by recrystallization after
reduction, leading to 9 in high ee. Iodocyclization followed by reduction
delivered the lactone 10, that was alkylated with high diastereocontrol to give
11. The addition of isopropenyl lithium followed by protection then completed
the assembly of 2.
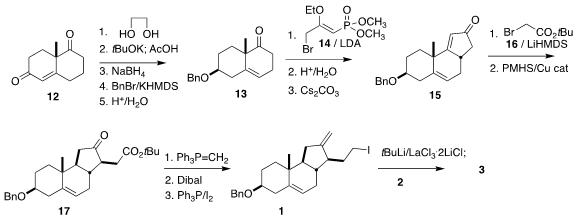
The tricyclic 1 was readily available from the commercial Wieland-Miescher
ketone 12.
Ketalization followed by deconjugation, reduction, protection and
deketalization delivered the ketone 13.
Alkylation with 14 followed by
hydrolysis and intramolecular
Horner-Wadsworth-Emmons cyclization led to the
cyclopentenone 15. Alkylation with 16 followed by
conjugate reduction gave the
ester 17. Methylenation followed by reduction and
Appel iodination then
completed the preparation of 1, that was coupled with 2 to give 3.
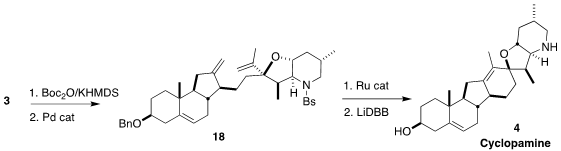
The Pd-mediated closure of the
tetrahydrofuran ring required some
experimentation. The carbonate derived from 3 proved successful, leading to
18.
Ring closing metathesis followed by reductive cleavage of the
protecting groups
then completed the synthesis of cyclopamine (4).
Nearly simultaneously, Shou-Fei Zhu of Nankai University and Shuanhu Gao of
East China Normal University described a very different route to cyclopamine 4
(J. Am. Chem. Soc. 2023, 145, 25086.
DOI: 10.1021/jacs.3c10362).
The synthesis of the Veratrum alkaloid
heilonine by Viresh H. Rawal of the University of Chicago, which addresses many
of the same issues, is also worth studying
(J. Am. Chem. Soc. 2021, 143, 16394;
DOI: 10.1021/jacs.1c08756,
![]() 2022, August 1).
2022, August 1).