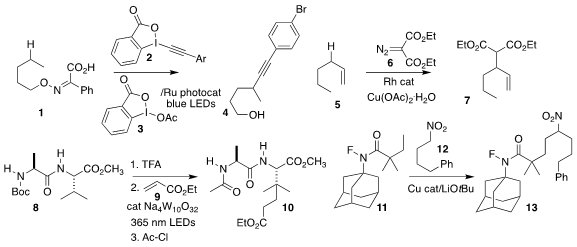
Yiyun Chen of the Shanghai Institute of Organic Chemistry used the
benziodoxole reagents 2 and 3 to convert the activated oxime 1
to the alkyne
4
(Org. Lett. 2022, 24, 5951.
DOI: 10.1021/acs.orglett.2c02210).
Masilamani Jeganmohan of the Indian Institute of
Technology Madras devised conditions for the insertion of the diazo malonate 6 into the allylic C-H of the alkene
5, leading to the diester 7
(Org. Lett. 1471260-52-2 web (4-Methoxyphenyl)methanol site 2023, 25, 1257.
DOI: 10.1021/acs.orglett.2c04356).
Takeru Torigoe and Yoichiro Kuninobu of Kyushu University deprotected
the urethane 8, then used the protonated amine to direct the coupling of ethyl
acrylate 9 with a distal C-H, to give 10
(Org. Lett. 2023, 25, 3708.
DOI: 10.1021/acs.orglett.3c01154).
Xiaojun Zeng of Nanchang University assembled the amide 13 by coupling the fluoroamide
11 with the nitroalkane 12
(Org. Lett. 2023, 25, 4632.
DOI: 10.1021/acs.orglett.3c01297).
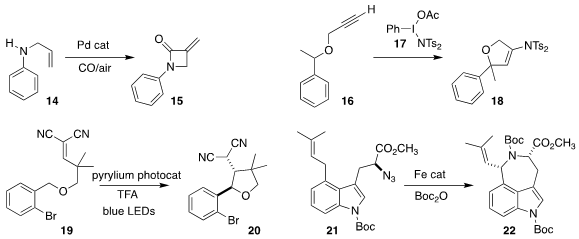
Matthew J. Gaunt and Alexei A. PMID:32926338 Lapkin of the University of Cambridge scaled
the flow carbonylation of the allyl amine 14
to the β-lactam 15
(Org. Process Res. Dev. 2023, 27, 649.
DOI: 10.1021/acs.oprd.2c00378).
Mi-hyun Kim of Gachon University used the hypervalent
iodine reagent 17 to convert the alkyne 16 to the corresponding alkylidene
carbene, leading to cyclization
to the dihydrofuran 18
(Org. Biomol. Chem. 2023, 21, 960.
DOI: 10.1039/D2OB02077G).
Devarajulu Sureshkumar of the Indian Institute of Science Education
and Research used a pyrilium photocatalyst to promote the cyclization of the
ether 19 to the tetrahydrofuran 20
(Synlett 2023, 34, 931.
DOI: 10.1055/a-1951-9897).
Yungen Liu of the Southern University of Science and Technology and Chi-Ming Che of Hong Kong University
used an iron porphyrin to catalyze the cyclization of the azide 21, leading
to the 3,4-azepinoindole 22
(Org. Chem. Front. 2023, 10, 1368.
DOI: 10.1039/D2QO01972H).
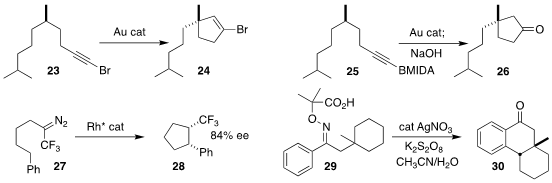
Pablo Barrio of the Universidad de Oviedo cyclized the bromoalkyne 23 to the
bromoalkene 24
(Angew. Chem. Int. Ed. 2023, 62, e202305296.
DOI: 10.1002/anie.202305296).
Zhuofeng Ke and Xinfang Xu of Sun Yat-sen University and Liming Zhang of the University of
California, Santa Barbara cyclized the alkynyl borane 25 to an intermediate that
was hydrolyzed to the ketone 26
(Angew. Chem. Int. Ed. 2023, 62, e202218175.
DOI: 10.1002/anie.202218175).
Professor Che used a Rh catalyst to cyclize the trifluorodiazoalkane 27
to the
cyclopentane 28
(Angew. Chem. Int. Ed. 2023, 62, e202215891.
DOI: 10.1002/anie.202215891).
Feng Li of Zaozhuang University, Fei Li of Liaoning Petrochemical University and Teng Wang
of Beihang University showed that the intermediate from the oxidation of the
oxime ether 29 led to the tricyclic ketone 30
(Org. Biomol. Chem. 2023, 21, 2700.
DOI: 10.1039/D2OB02329F).
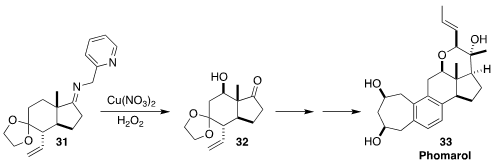
Phomarol (33) was isolated from a jellyfish-derived Phoma sp. fungus. En route
to 33, Jinghan Gui, also of the Shanghai Institute of Organic Chemistry,
oxidized the picolylimine 31 to the ketone 32, having the key C-12 OH
(J. Am. Chem. Soc. 2023, 145, 9354.
DOI: 10.1021/jacs.3c02817).