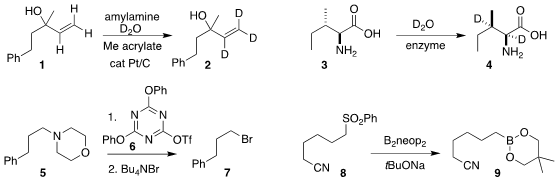
Kwihwan Park, Tsuyoshi Yamada, and Hironao Sajiki of Gifu Pharmaceutical
University devised conditions for the exchange of the allylic alcohol 1 with
D2O, to give the trideuterated allylic alcohol 2
(Org. Chem. Front. 2022, 9, 1986.
DOI: 10.1039/D2QO00177B).
Andrew R. Buller of the University of Wisconsin used an enzyme to effect
the deuteration of the amino acid 3, leading to the dideuterated 4
(J. Am. Formula of 2-(Difluoromethyl)pyridin-4-amine Chem. Soc. Price of Sulforaphane 2022, 144, 7327.
DOI: 10.1021/jacs.2c00608).
Munetaka Kunishima of Kanazawa University alkylated the
tertiary amine 5 with the triazene 6, then displaced the ammonium salt, leading
to the bromide 7
(Tetrahedron Lett. 2022, 93, 153692.
DOI: 10.1016/j.tetlet.2022.153692).
Jiefeng Hu, Udo Radius and Todd B. Marder of the Julius-Maximilians-Universität Würzburg
converted the sulfone 8 into the
boronate 9
(Chem. PMID:23558135 Eur. J. 2022, 28, e202103866,
DOI: 10.1002/chem.202103866;
Chem. Commun. 2022, 58, 395,
DOI: 10.1039/D1CC06144E).
Chi Zhang of Nankai University used the azidobenziodazolone 11 in conjunction
with triphenylphosphine to convert the secondary alcohol 10 to the
azide
11 with inversion of absolute configuration
(Chem. Eur. J. 2022, 28, e202200272.
DOI: 10.1002/chem.202200272).
Jinbo Hu of the Shanghai Institute of Organic Chemistry described a parallel investigation
(Nature Commun. 2022, 13, 2752.
DOI: 10.1038/s41467-022-30132-x).
Sabuj Kundu of the Indian Institute of Technology Kanpur used an Ir catalyst to
convert the azide 13 into
the tertiary amine 14
(J. Org. Chem. 2022, 87, 628.
DOI: 10.1021/acs.joc.1c02625).
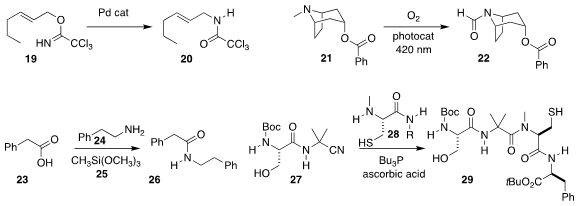
William J. Kruper of Michigan State University Midland effected Ritter opening of
the epoxide 15, leading to the protected amino alcohol 16
(J. Org. Chem. 2022, 87, 2063.
DOI: 10.1021/acs.joc.1c01475).
Peter R. Schreiner of Justus Liebig University established conditions for converting
the tertiary alcohol 17 into the azide 18
(Org. Lett. 2022, 24, 1460.
DOI: 10.1021/acs.orglett.2c00042).
Yusuke Kuroda of ITSUU Laboratory used a Pd catalyst to convert the imidate
19 into the allylic amide 20
(Synlett 2022, 33, 98.
DOI: 10.1055/s-0041-1737140).
Joshua P. Barham of the Universität Regensburg oxidized the tertiary amine 21 to the formamide 22
(Chem. Sci. 2022, 13, 1912.
DOI: 10.1039/D1SC05840A).
D. Christopher Braddock and Paul D. Lickiss of Imperial
College London used the nontoxic methyltrimethoxysilane to mediate the coupling
of the amine 24 with the carboxylic acid 23, leading to the
amide 26
(Org. Lett. 2022, 24, 1175.
DOI: 10.1021/acs.orglett.1c04265).
Yujiro Hayashi of Tohoku University assembled the congested amide 29 by
coupling the cysteine derivative 28 with the α-amidonitrile 27
(J. Am. Chem. Soc. 2022, 144, 10145.
DOI: 10.1021/jacs.2c02993).
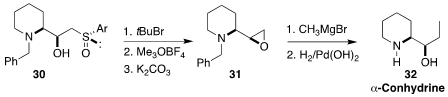
α-Conhydrine (32) is one of the poisonous alkaloids of hemlock, Conium maculatum.
In the course of a synthesis of 32, Xavier J. Salom-Roig of the Université de Montpellier reduced
the sulfoxide of 30 with t-butyl bromide, alkylated the resulting sulfide, and cyclized the
sulfonium salt to the epoxide 31
(Eur. J. Org. Chem. 2022, e202101451.
DOI: 10.1002/ejoc.202101451).