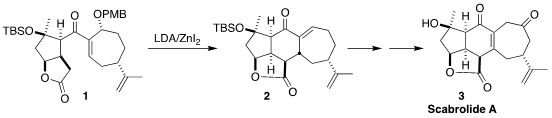
Scabrolide A (3), isolated from the soft coral Sinularia scabra,
has both anticancer and anti-inflammatory activity. PMID:24078122 En route to 3, David
Sarlah of the University of Illinois cyclized the enone 1 to the
lactone
2 (J. Am. Chem. Soc. 2-Bromo-3-fluoropyrazine In stock 2023, 145, 8805.
DOI: 10.1021/jacs.3c02317).
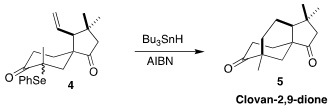
Based on computational analysis, Timothy R. Newhouse of Yale University
anticipated the successful free-radical cyclization of the selenide 4 to
clovan-2,9-dione (5)
(Nature Synth. 2023, 2, 527.
DOI: 10.1038/s44160-023-00271-0).
Zhi-Xiang Yu of Peking University described an alternative approach to 5
(Org. Lett. 728034-12-6 web 2017, 19, 6040,
DOI: 10.1021/acs.orglett.7b02656;
Org. Lett. 2022, 24, 5902,
DOI: 10.1021/acs.orglett.2c02111).
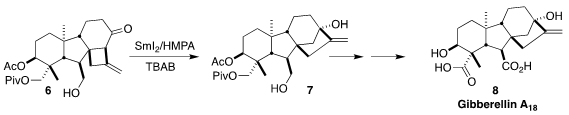
The gibberellins, represented by gibberellin A18 (8), regulate a
variety of plant development processes. Mingji Dai of Emory University developed
a concise route to 8 from commercial andrographolide, a key step of which
was the SmI2-mediated conversion of the cyclobutane 6 to the
tertiary alcohol 7
(J. Am. Chem. Soc. 2023, 145, 53.
DOI: 10.1021/jacs.2c12470).
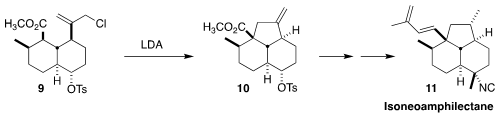
Isoneoamphilectane (11), isolated from the tropical marine sponge
Cymbastela hooperi, shows activity against Mycobacterium tuberculosis
H37Rv. Christopher D. Vanderwal of the University of California Irvine assembled
the unusual tricyclic skeleton of 11 by the cyclization of 9 to 10
(J. Am. Chem. Soc. 2023, 145, 3716.
DOI: 10.1021/jacs.2c13137).
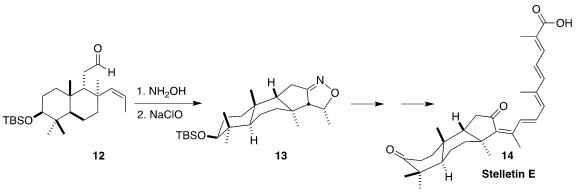
The stelletins, represented by stelletin E (14), isolated from sponges
of the genus Stelleta, show remarkable selective cytotoxicity. Xiaozhen
Jiao and Ping Xie of the Institute of Materia Medica established a practical
route to an advanced intermediate in the synthesis of 14, centered on the
intramolecular
dipolar cyclization
to 13 of the nitrile oxide derived from the aldehyde 12
(Org. Biomol. Chem. 2023, 21, 3850.
DOI: 10.1039/D3OB00319A).
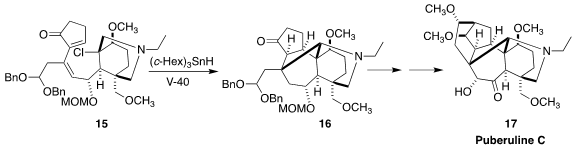
Puberuline C (17) was isolated from a traditional Chinese medical plant,
Aconitum barbatum var. puberulum. Masayuki Inoue of the University of Tokyo
assembled the ketone 16, having the pentacylic core of 17, by the cascade
radical cyclization of 15
(J. Am. Chem. Soc. 2023, 145, 600.
DOI: 10.1021/jacs.2c11259).