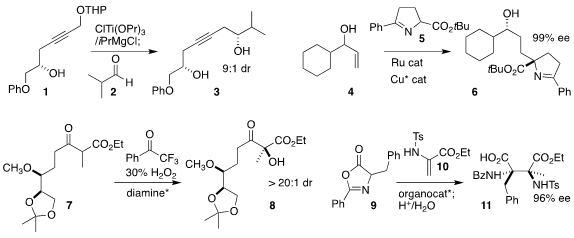
Jin Kun Cha of Wayne State University achieved high diastereoselectivity in
the reductive addition of the propargyl ether
1 to the aldehyde 2, to give the 1,6-diol 3
(Org. Lett. 2022, 24, 6252.
DOI: 10.1021/acs.orglett.2c02258).
Chun-Jiang Wang of Wuhan University
effected conjugate addition of
5 to the enone derived from the allylic alcohol
4, leading after in situ reduction to the 1,4-amino alcohol 6 in high ee
(Angew. Chem. Int. Ed. 2022, 61, e202206517.
DOI: 10.1002/anie.202206517).
Ya-Qiu Long of Soochow University prepared the protected 1,5-diol 7 by the enantioselective
oxidation of the β-keto ester 7
(Chem. Commun. 2022, 58, 13447.
DOI: 10.1039/D2CC04529J).
Ke-Qiang Hou and Xiao-Feng Xiong of Sun Yat-sen
University used (DHQD)2PHAL to mediate the construction of the 1,2-diamine 11
by the addition of the protected α-amino acid 9 to the ene sulfonamide 10
(J. Org. Chem. 2022, 87, 8709.
DOI: 10.1021/acs.joc.2c00950).
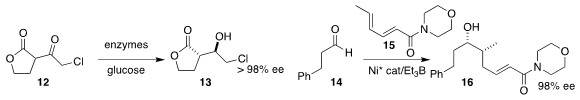
Todd K. PMID:24220671 2422999-74-2 custom synthesis Hyster of Cornell University used a combination of enzymes to reduce
the lactone 12 to the alcohol 13
(Org. 1003575-43-6 Price Process Res. Dev. 2022, 26, 2096.
DOI: 10.1021/acs.oprd.2c00017).
Alois Fürstner of the Max-Planck-Institut für Kohlenforschung achieved high ee in the
preparation of the alcohol 16 by the 1,2-addition of the dienyl amide 15 to the
aldehyde 14
(J. Am. Chem. Soc. 2022, 144, 18817.
DOI: 10.1021/jacs.2c09328).
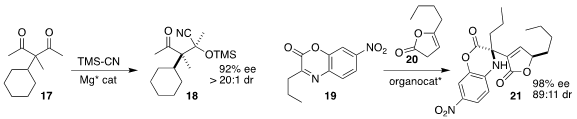
Zhongxing Huang of the University of Hong Kong converted the prochiral β-diketone 17 into the
cyanohydrin 18
(Angew. Chem. Int. Ed. 2022, 61, e202208443.
DOI: 10.1002/anie.202208443).
Jisheng Luo and Li Deng of Westlake University used a Cinchona-derived diamine to prepare 21 by the
addition of the lactone 20 to the imine 19
(JACS Au 2022, 2, 2678.
DOI: 10.1021/jacsau.2c00465).
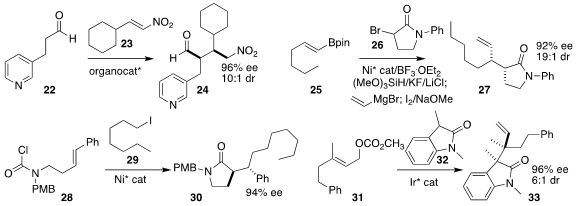
Helma Wennemers of ETH Zürich used a tripeptide to catalyze the enantioselective addition
of the aldehyde 22 to the nitroalkene 23, to give 24
(Adv. Synth. Catal. 2022, 364, 3354.
DOI: 10.1002/adsc.202200576).
Xile Hu of the Ecole Polytechnique Fédérale de
Lausanne added the α-bromolactam 26 to the alkenyl borane 25, leading, after the
addition of vinyl magnesium bromide, to the alkene 27
(Nature Catal. 2022, 5, 1180.
DOI: 10.1038/s41929-022-00894-0).
K. N. Houk of UCLA and Yifeng Chen of the East China University of
Science & Technology described the assembly of the
lactam 30 by the coupling of
the amide 28 with the alkyl iodide 29
(Angew. Chem. Int. Ed. 2022, 61, e202207536.
DOI: 10.1002/anie.202207536).
Yangbin Liu of Shenzhen Bay Laboratory and Xiaoming Feng of Sichuan
University constructed the vicinal quaternary stereogenic centers of the
oxindole
33 by the alkylation of the lactam 32 with the allylic alcohol 31
(Angew. Chem. Int. Ed. 2022, 61, e202208837.
DOI: 10.1002/anie.202208837).
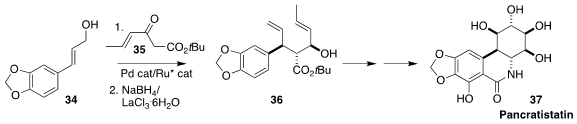
Pancratistatin (37), isolated from the bulbs of the Hawaiian spider lily
Hymenocallis littoralis, shows significant anticancer activity. Shinji Tanaka of
AIST and Masato Kitamura of Nagoya University alkylated the β-keto ester 35 with
the allylic alcohol 34, leading after reduction to the alcohol 36, having three
of the six stereogenic centers of 37
(Nature Commun. 2022, 13, 5876.
DOI: 10.1038/s41467-022-33432-4).