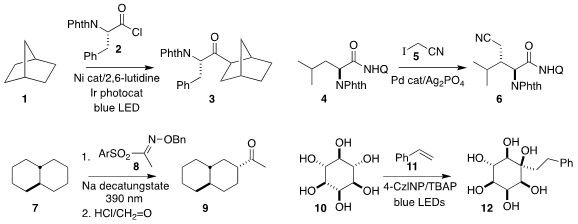
Soon Hyeok Hong of KAIST devised the photochemically-promoted
coupling of the acid chloride
2 with the hydrocarbon 1, leading to the ketone 3 as a mixture of diastereomers
(Nature Commun. tert-butyl (5-bromopentyl)carbamate Formula 2022, 13, 5200.
DOI: 10.1038/s41467-022-32851-7).
Devarajulu Sureshkumar of the
Indian Institute of Science Education and Research Kolkata developed the
diastereoselective preparation of the protected α-amino acid 6 by the coupling
of the amide 4 with iodoacetonitrile 5
(Chem. Commun. 2022, 58, 7793.
DOI: 10.1039/D2CC03106J).
Zhiqiang Pan and Chengfeng Xia of Yunnan University observed high regioselectivity in the
acetylation of trans-decalin (7) with the phenylsulfonyl ethanone oxime
8, leading after hydrolysis to the ketone 9
(Org. Lett. 2022, 24, 7983.
DOI: 10.1021/acs.orglett.2c03142).
Jian Liu and Xiaolei Wang of Lanzhou University achieved high site selectivity in the preparation of
the polyol 12 by coupling myo-inositol 10 with styrene 8
(Chem. Commun. Price of 1-Chloropyrrolo[1,2-c]pyrimidine 2022, 58, 9934.
DOI: 10.1039/D2CC03569C). PMID:23800738
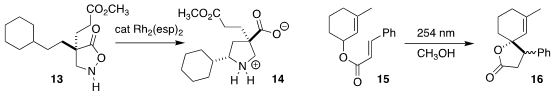
Hidetoshi Noda and Masakatsu Shibasaki of the Institute of Microbial
Chemistry also achieved high diastereoselectivity in the cyclization of the
isoxazolidin-5-one 13 to the
pyrrolidine 14
(Angew. Chem. Int. Ed. 2022, 61, e202212421.
DOI: 10.1002/anie.202212421).
Xin Sun and Miao Yu of Huanghuai University and Ji-Kai Liu and Bin Wu
of the South-Central University for Nationalities effected the photochemically-promoted
cyclization of the allyl cinnamate 15 to the
lactone 16
(Org. Chem. Front. 2022, 9, 2316.
DOI: 10.1039/D1QO01952J).
Josep M. Luis and Miquel Costas of the Universitat de Girona and Massimo Bietti of the
Università "Tor Vergata" showed that by choice of catalyst, either methyl group
of ketopinic acid (17) could be oxidized to the lactone, 18 or its regioisomer
(J. Am. Chem. Soc. 2022, 144, 19542.
DOI: 10.1021/jacs.2c08620).
Lung Wa Chung of the Southern University of Science and Technology and Zheng Huang of SIOC
achieved remarkable regioselectivity in the conversion of the silane 19 to the cyclic
silane 21, with t-butyl ethylene 20 as the H2 acceptor
(J. Am. Chem. Soc. 2022, 144, 20903.
DOI: 10.1021/jacs.2c09356).
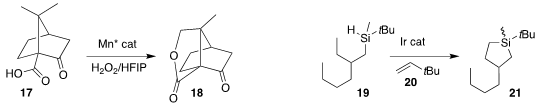
The Rh-catalyzed cyclization of the oxime tosylate 22 to the
cyclopropane 23
developed by Sailu Munnuri of the UT Southwestern Medical Center is thought to
proceed by hydride abstraction followed by recombination
(J. Am. Chem. Soc. 2022, 144, 17989.
DOI: 10.1021/jacs.2c07427).
The cyclization of the oxime aldehyde 24 to the
cyclobutanol
25 devised by Fabrizio Vetica and Patrizia Gentili of the Sapienza University of
Rome proceeds by H-atom abstraction/recombination
(J. Org. Chem. 2022, 87, 13803.
DOI: 10.1021/acs.joc.2c01503).
Lin Guo and Wujiong Xia of the Harbin Institute of Techology used a similar strategy to
assemble 28 by coupling the alkyne 26 with the α-keto acid 27
(Org. Chem. Front. 2022, 9, 6513.
DOI: 10.1039/D2QO01424F).
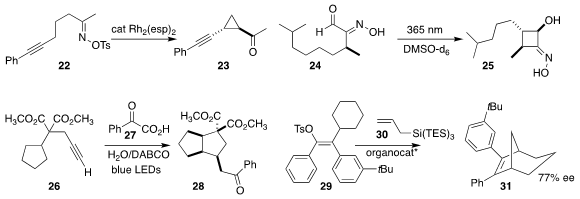
Matthew S. Sigman of the University of Utah and K. N. Houk and Hosea M. Nelson of UCLA achieved appreciable
enantioselectivity in the imidodiphosphorimidate-catalyzed cyclization, mediated
by the allyl silane 30, of the enol tosylate 29 to the bicyclic alkene 31
(Science 2022, 378, 1085.
DOI: 10.1126/science.ade5320).
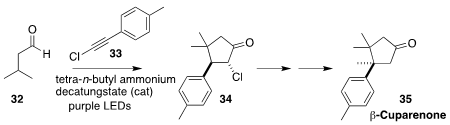
Gangguo Zhu of Zhejiang Normal University devised a concise synthesis of β-cuparenone
(35), based on the coupling of the aldehyde 32 with the chloroalkyne
33 to give 34.
Cyclopentanone
formation proceeds by initial formation of the acyl radical,
that adds regioselectively to the alkyne. The resulting alkenyl radical
abstracts an H-atom to give the tertiary radical, that then adds in a conjugate
sense to the enone, to give 34
(Nature Commun. 2022, 13, 4734.
DOI: 10.1038/s41467-022-32467-x).