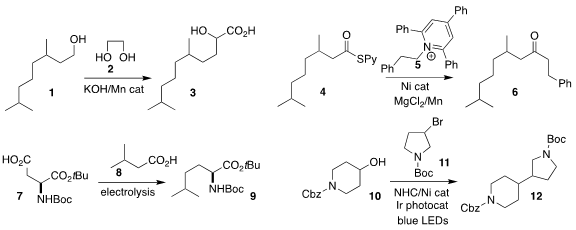
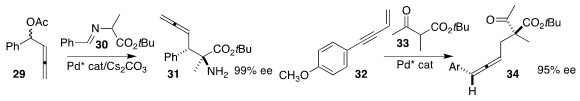Biplap Maji of the Indian Institute of Science Education and Research Kolkata
prepared the α-hydroxy acid 3 by combining the primary alcohol 1 with ethylene
glycol 2
(Angew. Chem. Formula of 117585-92-9 Int. Ed. 2023, 62, e202218329.
DOI: 10.1002/anie.202218329).
Ramagonolla Kranthikumar
of Harvard University assembled the ketone 6 by coupling the thioester 4 with
the pyridinium salt 5
(Org. Lett. 2023, 25, 3623.
DOI: 10.1021/acs.orglett.3c00943).
Yu Kawamata and Phil S. Baran
of Scripps/La Jolla established practical conditions for
Kolbe electrolysis,
illustrated by the cross coupling of the acid 7 with an excess of the acid
8, leading to 9
(Science 2023, 380, 81.
DOI: 10.1126/science.adf4762).
David W. C. PMID:23618405 MacMillan of Princeton
University devised the Ni catalyzed
coupling of the bromide 11 with the
secondary alcohol 10 to give 12
(J. Am. Chem. Soc. 2023, 145, 7736.
DOI: 10.1021/jacs.3c01488). 958451-91-7 site
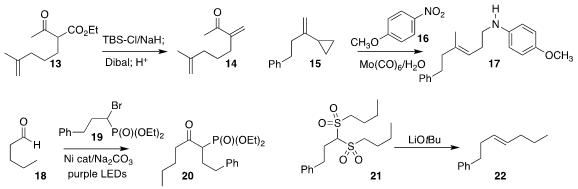
Xing-Wei Yang of Sun Yat-sen University optimized the conversion of the β-keto
ester 13 to the α-methylene ketone 14
(Chem. Commun. 2023, 59, 6215.
DOI: 10.1039/D3CC01268A).
Jin-Bao Peng of Wuyi University developed the reductive coupling of the nitroaromatic 16 with the alkenyl cyclopropane
15, leading to the homoallylic amine 17
(Org. Lett. 2023, 25, 2991.
DOI: 10.1021/acs.orglett.3c00781).
Tao Xu of Tongji University prepared the β-keto phosphonate 20 by coupling the bromophosphonate
19 with the aldehyde 18
(Org. Chem. Front 2023, 10, 3061.
DOI: 10.1039/D3QO00543G).
Manuel van Gemmeren of Westfälische Wilhelms-Universität Münster devised a
variation on the Ramberg-Bäcklund rearrangement, converting the
readily-available bis-sulfone 21 to the alkene 22
(Chem. Eur. J. 2023, 29, e202203512.
DOI: 10.1002/chem.202203512).
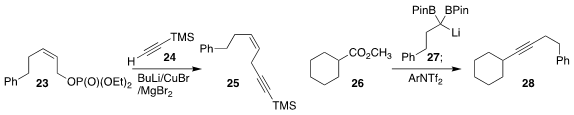
Narihito Ogawa of Meiji University showed that an allylic phosphonate such as
23 could be coupled with an alkyne 24 to give the eneyne 25 without scrambling the alkene geometry
(Eur. J. Org. Chem. 2023, 26, e202300056.
DOI: 10.1002/ejoc.202300056).
Chao Liu of the Lanzhou Institute of Chemical Physics established a powerful new method for
alkyne synthesis, combining the lithiated diborane 27 with the ester 26 to give
the alkyne 28
(Nature Synthesis 2023, 2, 413.
DOI: 10.1038/s44160-023-00243-4).
Shengming Ma of the Shanghai Institute of Organic Chemistry and Wanbing Zhang
of Shanghai Jiao Tong University achieved high enantioselectivity and
diasteroselectivity in the preparation of the α-quaternary amine 31 by the
alkylation of the protected amino ester 30 with the racemic allene 29
(Angew. Chem. Int. Ed. 2023, 62, e202218146.
DOI: 10.1002/anie.202218146).
Xueling Mi of Beijing Normal University and Sanzhong Luo of Tsinghua University also achieved high enantioselectivty in
the alkylation of the β-keto ester 33 with the eneyne 32 to give the allene 34
(Nature Commun. 2023, 14, 2911.
DOI: 10.1038/s41467-023-38488-4).
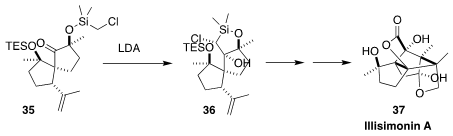
Illisimonin A (37) was isolated from the poisonous East Asian tree Illicium
simonsii. Markus Kalesse of Leibniz Universität Hannover, faced with the
challenge of adding a nucleophile to the very congested ketone of 35, solved the
problem by cyclizing the chloromethyl silane 35 to the tertiary alcohol 36
(J. Am. Chem. Soc. 2023, 145, 7021.
DOI: 10.1021/jacs.3c01262).