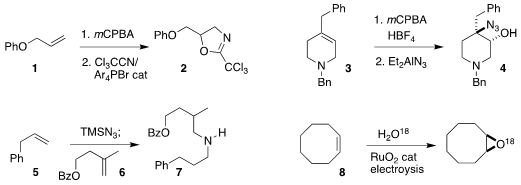
Yasunori Toda and Hiroyuki Suga of Shinshu University achieved high
regioselectivity in the conversion of the epoxide derived from 1 to the
oxazoline 2
(Chem. Commun. 3-(4-Fluorophenoxy)azetidine Order 2022, 58, 11819.
DOI: 10.1039/D2CC03782C).
David A. Candito of Merck Process
observed the opposite regioselectivity in the conversion of the epoxide derived
from 3 to the azido alcohol
4
(Org. Process Res. Dev. 2022, 26, 3034.
DOI: 10.1021/acs.oprd.2c00121).
Hao Wang and Fei Wang of Nankai University prepared the amine 7 by coupling the alkene
5 with the alkene 6
(J. Am. 8-Bromo-1,6-naphthyridine Chemical name Chem. Soc. 2022, 144, 16316.
DOI: 10.1021/jacs.2c07643).
Guohua Zhao of Tongji
University and Xin-Hao Li of Shanghai Jiao Tong University developed a Ru
catalyst for the electrochemical conversion of the alkene 8 to the
epoxide 9
(Angew. Chem. Int Ed. PMID:23789847 2022, 61, e202207108.
DOI: 10.1002/anie.202207108).
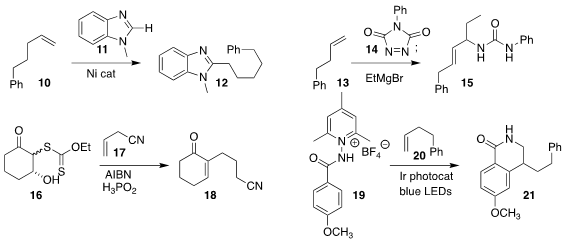
Hong-Mei Sun of Soochow University added the benzimidazole 11 to the alkene
10 to give the
benzimidazole
12, a protected form of the carboxylic acid
(Org. Lett. 2022, 24, 8875.
DOI: 10.1021/acs.orglett.2c03689).
Yusuke Kuroda of Kyoto University prepared the amide 15 by coupling the alkene
13, the triazoledione 14, and ethyl magnesium bromide
(Org. Lett. 2022, 24, 6224.
DOI: 10.1021/acs.orglett.2c02585).
Samir Z. Zard of the Ecole Polytechnique added the xanthate 16 to the alkene
17, leading, after dehydration, to the
2-alkyl cyclohexenone 18
(Org. Lett. 2022, 24, 6973.
DOI: 10.1021/acs.orglett.2c03671).
Pan-Pan Zhou and Wei Yu of Lanzhou University assembled the
lactam 21 by stitching together the alkene
20 with the N-aminopyridinium salt 19
(Org. Lett. 2022, 24, 6037.
DOI: 10.1021/acs.orglett.2c02323).
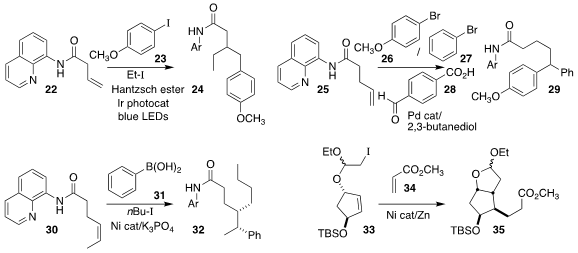
Biplab Maji of the Indian Institute of Science Education and Research Kolkata
constructed the amide 24 by the three-component coupling of the aryl iodide
23, ethyl iodide, and the alkene 22
(Org. Lett. 2022, 24, 6261.
DOI: 10.1021/acs.orglett.2c02355).
In an alternate three-component coupling, Xiaojin Wu of Nanjing Tech University prepared the
amide 29 by combining the aryl bromide 26 and the aryl bromide 27 with the
alkene 25, in the presence of the promoter 28
(Org. Lett. 2022, 24, 6520.
DOI: 10.1021/acs.orglett.2c02416).
Wei Guan of Northeast Normal University and Chao Wang of Tianjin Normal University
observed high diastereoselectivity in the coupling of the areneboronic acid 31 with 1-iodobutane and the alkene
30
(Org. Chem. Front. 2022, 9, 6556.
DOI: 10.1039/D2QO01182D).
Mingji Dai of Purdue University also observed high diastereoselectivity in the coupling
of the iodoacetal 33 with methyl acrylate 34, leading to the ester
35
(J. Org. Chem. 2022, 87, 8796.
DOI: 10.1021/acs.joc.2c00770).
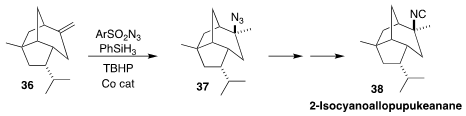
2-Isocyanoallopupukeanane (38) was isolated from the nudibranch Phyllidiella
putulosa. In the end game of a synthesis of 38, Keiji Tanino of Hokkaido
University achieved high diastereoselectivity in the conversion of the alkene 36
to the azide 37 following the Carreira protocol
(Org. Lett. 2022, 24, 6407.
DOI: 10.1021/acs.orglett.2c02434).