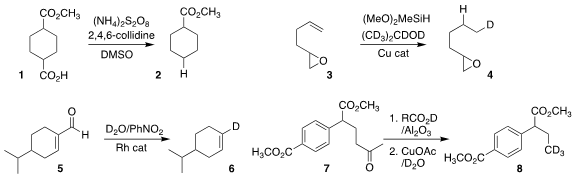
Ai-Lan Lee of Heriot-Watt University developed a protocol for the
decarboxylation of the carboxylic acid
1 to the hydride 2
(Org. Lett. 2022, 24, 686.
DOI: 10.1021/acs.orglett.1c04079).
Joseph R. Clark of Marquette University
reduced the alkene
3 to the monodeuterated 4
(Chem. Eur. J. 2022, 28, e202104340.
DOI: 10.1002/chem.202104340).
Qing-An Chen of the Dalian Institute of Chemical Physics decarbonylated the α,β-unsaturated aldehyde
5 to the monodeuterated alkene 6
(J. Am. Chem. Oxetan-3-yl trifluoromethanesulfonate Chemscene Soc. PMID:23290930 2022, 144, 11081.
DOI: 10.1021/jacs.2c04422).
Guangbin Dong of the University of Chicago showed that it was possible to
cleave a methyl ketone 7
to leave the mono-, di-, or, as illustrated, trideuterated hydrocarbon 8
(J. Am. Chem. 1846598-27-3 Chemscene Soc. 2022, 144, 9570.
DOI: 10.1021/jacs.2c04382).
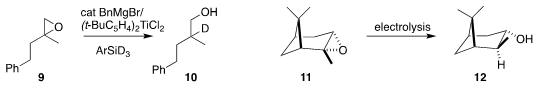
Andreas Gansäuer of the Universität Bonn
reduced the epoxide
9 to the monodeuterated alcohol 10
(Angew. Chem. Int. Ed. 2022, 61, e202114198.
DOI: 10.1002/anie.202114198).
Xiaotian Qi and Qingquan Lu of Wuhan University used electrolysis to
reduce the pinene-derived epoxide 11 to the secondary alcohol 12
(J. Am. Chem. Soc. 2022, 144, 1389.
DOI: 10.1021/jacs.1c11791).
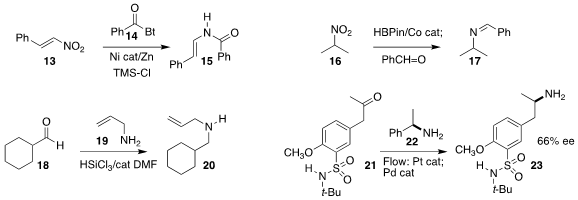
Wanfang Li of the University of Shanghai for Science and Technology
reduced
the nitroalkene 13 in the presence of the N-acyl benzotriazole
14, to give directly the enamide 15
(Org. Lett. 2022, 24, 58.
DOI: 10.1021/acs.orglett.1c03535).
Davit Hayrapetyan and Andrey Y. Khalimon of Nazarbayev University showed that the intermediate from the
Co-catalyzed reduction of the nitro alkane 16 could be condensed with an
aldehyde, leading to the imine 17 that could be reduced to the secondary amine
(Adv. Synth. Catal. 2022, 364, 601.
DOI: 10.1002/adsc.202101043).
Pavel Kočovsky of Charles University devised mild
conditions for the reductive amination of the aldehyde
18 with allylamine 19 to give the N-allyl amine 20
(J. Org. Chem. 2022, 87, 920.
DOI: 10.1021/acs.joc.1c01561).
Shū Kobayashi of the
University of Tokyo developed a flow protocol for the intial diastereoselective
reductive amination of the ketone 21 with the enantiomerically-pure secondary
amine 22, followed by reductive debenzylation, to give the enantio-enriched
product amine 23 directly
(Angew. Chem. Int. Ed. 2022, 61, e202115643.
DOI: 10.1002/anie.202115643).
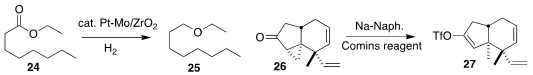
Tomoo Mizugaki of Osaka University optimized a Pt/Mo/ZrO2 catalyst for the
reduction of an ester 24
to the ether 25
(JACS Au 2022, 2, 665.
DOI: 10.1021/jacsau.1c00535).
Even in the presence of two alkenes, Rongbiao Tong of the Hong Kong University
of Science and Technology was able to reduce the cyclopropyl ketone 26 with Na/naphthalene
and trap the resulting enolate as the enol triflate 27
(Angew. Chem. Int. Ed. 2022, 61, e202115384.
DOI: 10.1002/anie.202115384).
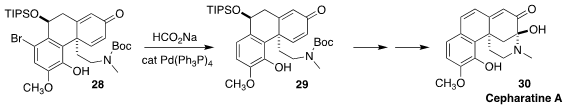
Cepharatine A (30), isolated from the Chinese vine Stephania cepharantha,
showed bacteriostatic activity. En route to 30, Minami Odagi and Kazuo Nagasawa
of the Tokyo University of Agriculture and Technology were able to reductively
remove the bromide of 28 to give
29, without interference by the two readily-reduced alkenes
(J. Org. Chem. 2022, 87, 1065.
DOI: 10.1021/acs.joc.1c02371).