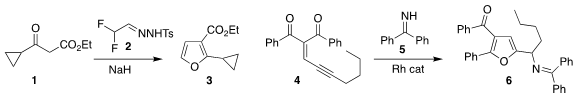
Jin-Na Song of Jilin University and Yongquan Ning of Northeast Normal
University assembled the
furan
3 by combining the diazoalkane derived in situ
from the tosylhydrazone 2 with the β-keto ester 1
(Org. PMID:24563649 Chem. 3-Azidopropylamine web Front. 2022, 9, 5825.
DOI: 10.1039/D2QO01045C).
Ju Hyun Kim of Gyeongsang National University prepared the furan 6 by
coupling the eneyne 4 with the benzophenone imine 5
(J. Org. Chem. 2022, 87, 16424.
DOI: 10.1021/acs.joc.2c02119).
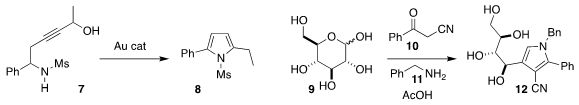
Debayan Sarkar of the Indian Institute of Technology Indore used gold
catalysis to cyclize the propargyl alcohol 7 to the
pyrrole 8
(J. Org. Chem. 2022, 87, 9729.
DOI: 10.1021/acs.joc.2c00780).
Zaher M. A. Judeh of Nanyang Technological University combined the β-keto nitrile
10 and benzylamine 11 with glucose 9, leading to the pyrrole 12
(J. Org. 1346809-61-7 site Chem. 2022, 87, 12115.
DOI: 10.1021/acs.joc.2c01270).
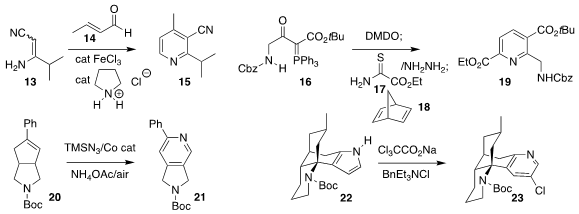
Xi-Jie Dai of Amgen coupled the cyano enamine 13 with crotonaldehyde 14,
leading to the pyridine 15
(J. Org. Chem. 2022, 87, 8437.
DOI: 10.1021/acs.joc.2c00576).
David Sarlah of the University of Illinois oxidized the phosphorane 16, then combined that product
with the thioamide 17, leading, after reaction with 18, to the pyridine 19
(Angew. Chem. Int. Ed. 2022, 61, e202212299.
DOI: 10.1002/anie.202212299).
Hao Wei of Northwest University expanded
the cyclopentene 20 to the pyridine 21
(J. Am. Chem. Soc. 2022, 144, 22433.
DOI: 10.1021/jacs.2c10570).
Mingji Dai of Purdue University expanded the pyrrole 22 to the pyridine 23
(J. Am. Chem. Soc. 2021, 143, 16383.
DOI: 10.1021/jacs.1c08626).
Timothée Constantin of the University of Manchester and Daniele Leonori of
RWTH Aachen University reacted the iodide 25 with the diazonium salt 24, leading
to the indole 26
(Org. Lett. 2022, 24, 7883.
DOI: 10.1021/acs.orglett.2c02840).
Jun-Long Niu and Mao-Ping Song of Zhengzhou University oxidized the aniline derivative
27 with the reagent 28 to give the indole 29
(Org. Chem. Front. 2022, 9, 3723.
DOI: 10.1039/D2QO00562J).
Roberto Sanz of the Universidad de Burgos cyclized the alkyne 30 to the indole 31
(Adv. Synth. Catal. 2022, 364, 3716.
DOI: 10.1002/adsc.202200824).
Junichiro Yamaguchi of Waseda University alkynylated the sulfonamide 32
with the reagent 33, then cyclized that product to the indole 34
(Synthesis 2022, 54, 4963.
DOI: 10.1055/a-1786-9881).
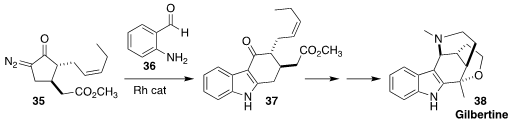
The alkaloid gilbertine (38) was isolated from Aspidosperma gilbertii of the American
tropics. En route to 38, Bei Wei and Liansuo Zu of Tsinghua University coupled the methyl
jasmonate-derived diazoketone 35 with the aldehyde 36, leading to the indole 37
(Org. Lett. 2022, 24, 7320.
DOI: 10.1021/acs.orglett.2c02778).