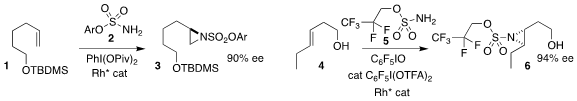
Benjamin Darses of the Université Grenoble-Alpes and Marie Sircoglou and
Philippe Dauban of the Université Paris-Saclay effected the enantioselective
aziridination of the terminal alkene
1 with 2, leading to 3
(J. Am. Chem. Soc. 2022, 144, 17156.
DOI: 10.1021/jacs.2c07337).
Robert J. Phipps of the University of Cambridge described the complementary aziridination
of the internal alkene 4 with 5, to give 6
(J. Buy4-Methoxycarbonyl-3-methyl-benzoicacid Am. Chem. Soc. 2023, 145, 7516.
DOI: 10.1021/jacs.3c00693).
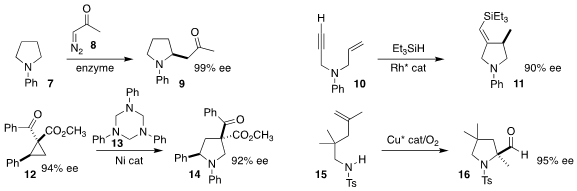
Rudi Fasan of the University of Rochester optimized the preparation of the
ketone 9 by the enzymatic coupling of the diazo ketone 8 with the
pyrrolidine 7
(J. Am. PMID:35126464 Chem. Soc. 1427158-38-0 custom synthesis 2023, 145, 537.
DOI: 10.1021/jacs.2c10775).
Frances H. Arnold of Caltech reported a parallel investigation
(Nature Catal. 2023, 6, 152.
DOI: 10.1038/s41929-022-00908-x).
Yingtang Ning and Fen-Er Chen of Fudan University effected the enantioselective
cyclization of the enyne 10 to the pyrrolidine 11
(Chem. Sci. 2023, 14, 4641.
DOI: 10.1039/D2SC06349B).
Michael P. Doyle of the University of Texas at San Antonio prepared the pyrrolidine
14 by coupling the activated cyclopropane 12 with 13
(Org. Lett. 2023, 25, 3029.
DOI: 10.1021/acs.orglett.3c00831).
Sherry R. Chemler of the State University of New York at Buffalo developed the oxidative
cyclization of the alkene 15 to the pyrrolidine 16
(J. Am. Chem. Soc. 2023, 145, 13715.
DOI: 10.1021/jacs.3c01985).
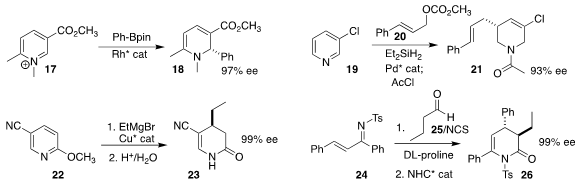
Rashad R. Karimov of Auburn University devised the enantioselective
conversion of the pyridinium salt 17 to the
dihydropyridine
18
(J. Am. Chem. Soc. 2023, 145, 11781.
DOI: 10.1021/jacs.3c03048).
Brian M. Stoltz, also of Caltech, prepared the tetrahydropyridine 21 by
coupling the pyridine 19 with the allylic carbonate 20
(J. Am Chem. Soc. 2023, 145, 11907.
DOI: 10.1021/jacs.3c02470).
Syuzanna R. Harutyunyan of the University
of Groningen effected enantioselective Grignard addition to the pyridine 22, leading after hydrolysis to the lactam
23
(Angew. Chem. Int. Ed. 2023, 62, e202217328.
DOI: 10.1002/anie.202217328).
Qian Liu of Guizhou University assembled the
lactam 26 by coupling
the aldehyde 25 with the imine 24
(J. Org. Chem. 2023, 88, 2404.
DOI: 10.1021/acs.joc.2c02821).
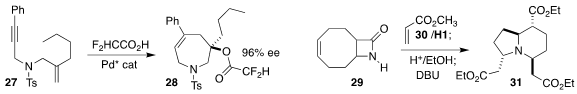
Linjun Qi of Taizhou University and Xiaofeng Tong of Changzhou University
cyclized the enyne 27 to the tetrahydroazepine 28
(J. Am. Chem. Soc. 2023, 145, 1973.
DOI: 10.1021/jacs.2c12756).
Loránd Kiss of the Institute of Organic Chemistry, Budapest prepared the
indolizine
31 by ring opening metathesis of the cyclooctene
29 with methyl
acrylate 30, followed by cyclization
(Synlett 2023, 34, 163.
DOI: 10.1055/s-0042-1751388).
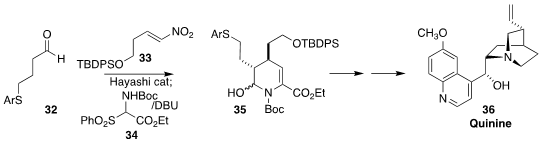
Quinine (36), in addition to its importance as an anti-malarial agent, has played an outsized role both as an organocatalyst,
and as a ligand for enantioselective transition-metal catalyzed transformations. Yujiro Hayashi of Tohoku University
devised a five-pot synthesis of 36, starting with the preparation of 35 by the sequential combination of
32, 33 and 34
(Nature Commun. 2022, 13, 7503.
DOI: 10.1038/s41467-022-34916-z).