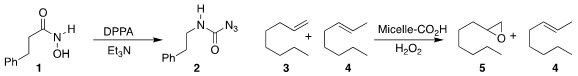
Masato Matsugi of Meijo University prepared the carbamoyl azide 2 by reaction
of the hydroxamic acid 1 with diphenyl phosphorazidate
(Tetrahedron Lett. PMID:32261617 2022, 95, 153727.
DOI: 10.1016/j.tetlet.2022.153727).
Yan Zhao of Iowa State University designed a catalyst that effected
epoxidation of 1-octene 3 to
5, leaving 2-octene (4) unreacted
(ACS Catal. 2022, 12, 3444.
DOI: 10.1021/acscatal.2c00253).
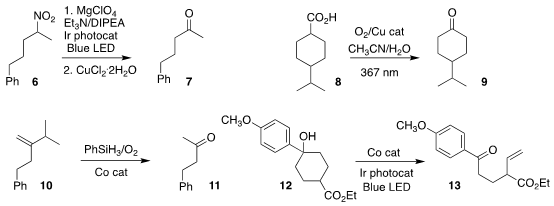
Bor-Cherng Hong of National Chung Cheng University devised a mild protocol
for converting the nitroalkane 6 to the
ketone 7
(Org. Biomol. tert-Butyl (2-iodoethyl)carbamate Order Chem. 2022, 20, 3292.
DOI: 10.1039/D2OB00267A).
Felix N. Castellano of North Carolina State University and Julia Rehbein
and Oliver Reiser of the University of Regensburg oxidized the carboxylic acid
8 to the ketone 9
(Chem. Commun. 2022, 58, 4456.
DOI: 10.1039/D2CC00570K).
Xiao-Yu Liu and Yong Qin of Sichuan University prepared the ketone 11 by the
oxidative cleavage of the
alkene 10
(Org. Chem. 14150-94-8 web Front. 2022, 9, 2135.
DOI: 10.1039/D2QO00125J).
Xuesong Wong of the Huazhong University of Science and Technology developed the photochemical conversion of
the tertiary benzylic alcohol 12 to the ω-alkenyl ketone 13
(ACS Catal. 2022, 12, 3710.
DOI: 10.1021/acscatal.2c00204).
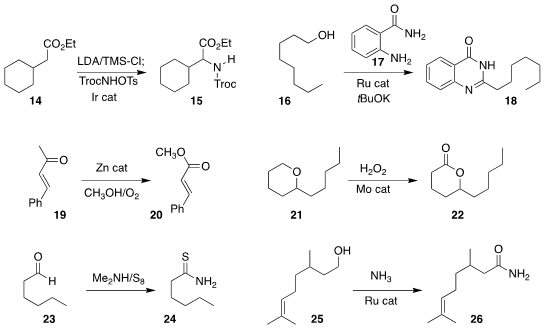
Sukbok Chang of KAIST effected the
amination of the ester
14 to give the α-amino ester 15
(Org. Lett. 2022, 24, 1088.
DOI: 10.1021/acs.orglett.1c04376).
Dipankar Srimani of the Indian Institute of Technology Guwahati assembled the
quinazolinone
18 by the borrowed hydrogen combination of the benzamide 17 with the alcohol 16
(J. Org. Chem. 2022, 87, 5556.
DOI: 10.1021/acs.joc.1c02913).
Zehui Zhang of the South-Central University for Nationalities
and Buxing Han of the Institute of Chemistry of the Chinese Academy of Sciences
oxidized the enone 19 to the
methyl ester 20
(Nature Commun. 2021, 12, 4823.
DOI: 10.1038/s41467-021-25118-0).
Dillip Kumar Chand of the Indian Institute of Technology Madras oxidized the
cyclic ether 21 to the
lactone 22
(J. Org. Chem. 2022, 87, 4061.
DOI: 10.1021/acs.joc.1c02855).
Guddeangadi N. Gururaja of the Central University of Gujarat converted the aldehyde 23 into the
thioamide 24
(J. Org. Chem. 2022, 87, 2410.
DOI: 10.1021/acs.joc.1c02307).
David Milstein of the Weizmann Institute of Science observed hydrogen evolution on the coupling of the alcohol
25 with ammonia to give the
amide 26
(Chem. Sci. 2022, 13, 3894.
DOI: 10.1039/D1SC07102E).
Zi-Qiang Rong of the Northwestern Polytechnical University reported a parallel investigation
(Org. Chem. Front. 2022, 9, 1703.
DOI: 10.1039/D2QO00004K).
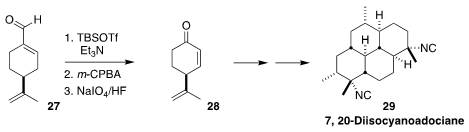
The tetracyclic bis-isonitrile 7,20-diisocyanoadociane (29), isolated from a
marine sponge, shows nanomolar antiplasmodial activity. Christopher D. Vanderwal
of the University of California, Irvine prepared dehydrocryptone 28, the starting material for a synthesis of
29, by the Razdan oxidation of commercial perillaldehyde 27
(J. Org. Chem. 2022, 87, 1398.
DOI: 10.1021/acs.joc.1c02700).