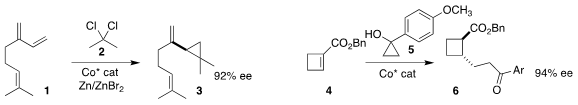
Christopher Uyeda of Purdue University achieved high enantioselectivity in
the cyclopropanation
of myrcene (1) with 2,2-dichloropropane (2) to give 3
(J. Am. Chem. Soc. 2023, 145, 9441.
DOI: 10.1021/jacs.3c01949).
Qinglei Chong and Fanke Meng of the Shanghai Institute of Organic Chemistry assembled the
cyclobutane 6 by conjugate addition
of the cyclopropylcarbinol 5 to the cyclobutene ester 4
(J. Am. Chem. Soc. Buy2-Bromo-4-chloro-6-methoxypyridine 2023, 145, 3588.
DOI: 10.1021/jacs.2c12475).
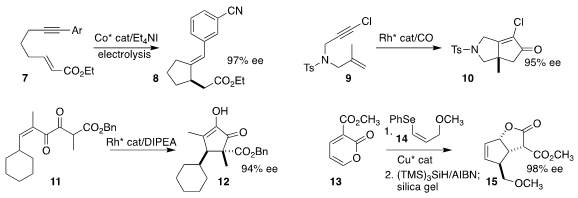
Junfeng Yang and Junliang Zhang of Fudan University used electrolysis to
cyclize the enyne 7 to the
cyclopentane 8
(Nature Commun. 2023, 14, 1301.
DOI: 10.1038/s41467-023-36704-9).
P. Andrew Evans of Queens University developed the Rh-catalysed
Pauson-Khand
cyclization of the chloroacetylene 9, leading to the
cyclopentenone 10
(Angew. PMID:24856309 Chem. Int. Ed. 2023, 62, e202300211.
DOI: 10.1002/anie.202300211).
Marcus A. Tius of the University of Hawaii effected the
Nazarov cyclization
of the α-diketone 11 to give 12 with high diastereoselectivity
(Org. Biomol. Buy2-Methylindole-4-carboxaldehyde Chem. 2023, 21, 5014.
DOI: 10.1039/D3OB00735A).
Quan Cai, also of Fudan University, carried out the
Diels-Alder cycloaddition of the alkenyl
selenide 14 to the 2-pyrone 13, leading, after reduction and acid-mediated
rearrangement, to the lactone 15
(Synlett 2023, 34, 948.
DOI: 10.1055/a-1990-5276).
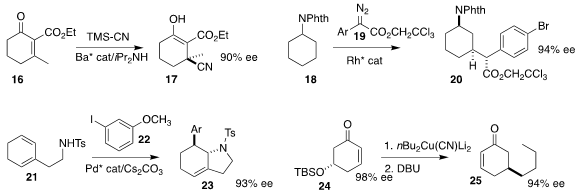
Xiaohua Liu and Xiaoming Feng of Sichuan University prepared the keto nitrile
17 by enantioselective conjugate addition of cyanide to the doubly-activated
cyclohexenone 16
(ACS Catal. 2023, 13, 877.
DOI: 10.1021/acscatal.2c05509).
Huw M. L. Davies of Emory University coupled the diazo ester 19 with the protected cyclohexylamine
18 to give 20 with high regio-, enantio- and diastereoselectivity
(Org. Lett. 2023, 25, 3995.
DOI: 10.1021/acs.orglett.3c00844).
Professor Zhang and Zhiming Li, also of Fudan University, and
Yuanjing Xiao of East China Normal University prepared the
cyclohexene 23 by
coupling the cyclohexadiene 21 with the iodoarene 22
(Angew. Chem. Int. Ed. 2023, 62, e202215407.
DOI: 10.1002/anie.202215407).
Twenty-five years ago, Fumie Sato of the Tokyo Institute
of Technology showed that the easily-prepared silyl ether 24 could readily be
converted to either enantiomer of the 5-alkyl cyclohexenone 25
(Angew. Chem. Int. Ed. 1998, 37, 2099.
DOI: 10.1002/(SICI)1521-3773(19980817)37:15<2099::AID-ANIE2099>3.0.CO;2-U).
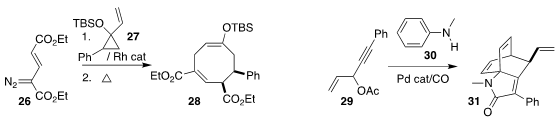
Paola Nava and Gaëlle Chouraqui of Aix Marseille University used the diazo
ester 26 to cyclopropanate the protected cyclopropylcarbinol 27, then heated the
product, leading to the cyclooctadiene 28
(Adv. Synth. Catal. 2023, 365, 1002.
DOI: 10.1002/adsc.202300015).
Yang Li, Qing Sun and Jin-Heng Li of Nanchang Hangkong University constructed
the lactam 31 by the
three-component coupling of the enyne 29, the aniline 30 and carbon monoxide
(Org. Lett. 2023, 25, 4303.
DOI: ).
Giovanni Maestri of the Università di Parma reported a parallel investigation
(Angew. Chem. Int. Ed. 2023, 62, e202216817.
DOI: 10.1002/anie.202216817).
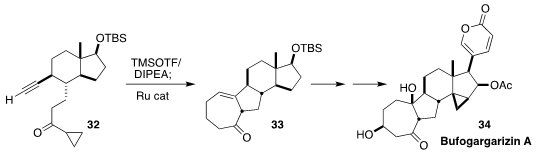
Bufogargarizin A (34) was isolated from the venom of the Asiatic toad
Bufo bufo gargarizins, long used in traditional Chinese medicine. Chuang-Chuang Li of the
Southern University of Science and Technology assembled the carbocyclic core of
34 by the cyclization of the alkyne 32 to the
cycloheptenone 33
(J. Am. Chem. Soc. 2023, 145, 2098.
DOI: 10.1021/jacs.2c13494).