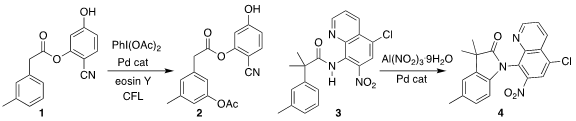
Haibo Ge of Texas Tech University and Debabrata Maity of the Indian Institute
of Technology Bombay effected the m-acetoxylation of the ester 1, leading to
2
(JACS Au 2023, 3, 1790.
DOI: 10.1021/jacsau.3c00231).
Dauquan Tu of Jiangsu Hansoh Pharmaceutical Co. and Jun
Luo and Chao Jiang of the Nanjing University of Science and Technology cyclized
the amide 3 to the lactam 4
(Org. Chem. Front. PMID:32472497 Buy2454490-66-3 2023, 10, 109.
DOI: 10.1039/D2QO01562E).
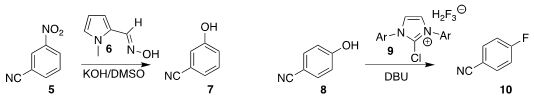
Michael J. 4-Bromo-3,6-dichloropyridazine In stock James of the University of Manchester used the oxime 6 to convert
the nitroaromatic 5 to the phenol 7
(Chem. Eur. J. 2023, 29, e202203807.
DOI: 10.1002/chem.202203807).
Gaper Tavčar of the Joef Stefan Institute prepared the
fluoroaromatic 10 by the
deoxyfluorination of the phenol 8 with the imidazolium 9
(Org. Lett. 2023, 25, 3649.
DOI: 10.1021/acs.orglett.3c01018).
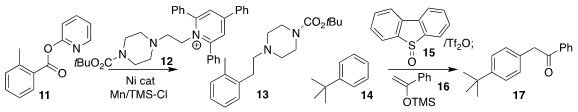
Daniel J. Weix of the University of Wisconsin coupled the benzoate 11 with
the pyridinium salt 12, leading to the
alkylated benzene derivative 13
(J. Am. Chem. Soc. 2023, 145, 9951.
DOI: 10.1021/jacs.2c11552). David J. Procter, also of the University of
Manchester, used blue light to promote the
assembly of the ketone 17 by the
coupling of the silyl enol ether 16 with the triaryl sulfonium salt derived from
the combination of 14 and 15
(Nature Chem. 2023, 15, 43.
DOI: 10.1038/s41557-022-01092-y).
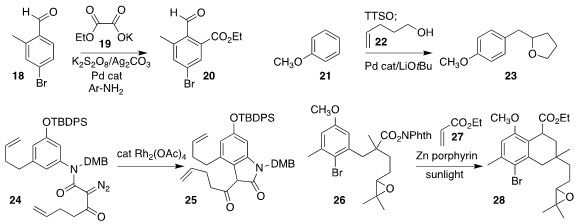
Sébastien Prévost of the Ecole Polytechnique employed a transient directing
group strategy to prepare the ester 20, coupling the aldehyde 18 with the
oxalate salt 19
(Org. Lett. 2023, 25, 1380.
DOI: 10.1021/acs.orglett.3c00086).
Ruzhang Liu of Yangzhou University
prepared the tetrahydrofuran 23 by combining the unsaturated alcohol 22 with the
triarylsulfonium salt derived from 21
(Org. Lett. 2023, 25, 2606.
DOI: 10.1021/acs.orglett.3c00582).
Xuegong She of Lanzhou University cyclized the amide 24 to the lactam 25
(Org. Lett. 2023, 25, 1003.
DOI: 10.1021/acs.orglett.3c00132).
Takehiko Yoshimitsu of Okayama University used sunlight to promote the
coupling of the N-hydroxyphthalimide ester 26 with ethyl acrylate
27, leading to the ester 28
(J. Org. Chem. 2023, 88, 1085.
DOI: 10.1021/acs.joc.2c02552).
Daniele Leonori of RWTH Aachen University oxidized the β-ketoester 29 to the methyl salicylate 30
(Angew. Chem. Int. Ed. 2023, 62, e202301656.
DOI: 10.1002/anie.202301656).
Christopher J. T. Hyland and Stephen G. Pyne of the University of Wollongong and Scott G.
Stewart of the University of Western Australia cyclized the enediyne 31 to the
dihydroisoindole 32
(J. Org. Chem. 2023, 88, 5391.
DOI: 10.1021/acs.joc.2c03040).
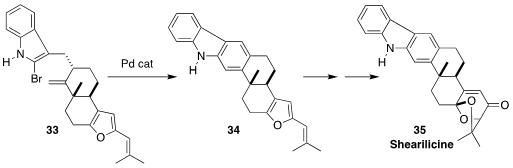
The carbazole alkaloid shearilicine (35), isolated from an endophytic
Penicillium species, showed interesting selective cytotoxicity. Timothy R.
Newhouse of Yale University constructed 34, with the central benzene ring of
35, by cyclizing the bromoindole 33
(J. Am. Chem. Soc. 2023, 145, 4394.
DOI: 10.1021/jacs.2c13584).