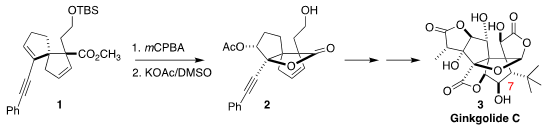
The ginkgolides, in particular ginkgolide B, lacking the OH at C-7, are
potent antagonists of platelet-aggregating acceptor (PAFR). PMID:24275718 Louis Barriault of
the University of Ottawa described the first synthesis of ginkgolide C (3), the most highly oxygenated of the family, using a route that in fact could be used
to prepare any of the ginkgolides. A key step in the synthesis was the
conversion of the bicyclic ester 1 to the
lactone 2
(J. Am. Formula of Benzo[d]oxazole-7-carbaldehyde Chem. Soc. 2022, 144, 17792.
DOI: 10.1021/jacs.2c08351). Fmoc-Ala-OH In stock
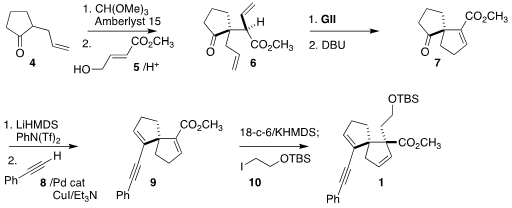
The starting materials for the synthesis were both commercially-available, the
cyclopentanone 4 and the allylic alcohol 5. Following the protocol of Daub,
acid-mediated combination of 4 with 5 led, via enol ether formation and Claisen
rearrangement, to the ester 6 with high regio-and diastereocontrol.
Ring-closing
metathesis followed by base treatment delivered the bicyclic ester 7. Conversion
to the enol triflate followed by coupling with the alkyne 8 led to 9, that was
alkylated with the iodide 10 to complete the preparation of 1.
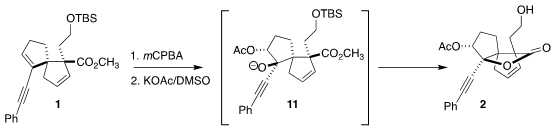
Epoxidation of 1 with
mCPBA followed by opening of the major diastereomer with
KOAc led, via the alkoxide 11, to the lactone 2. The
silyl group was lost in the course of the reaction sequence. The minor diastereomer from
mCPBA epoxidation spontaneously cyclized to the desired lactone.
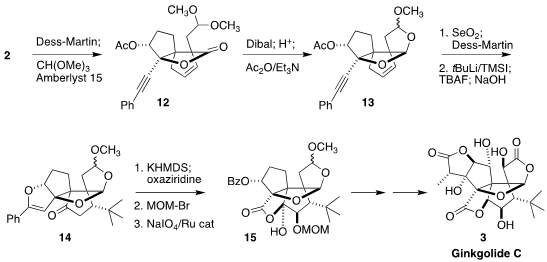
Oxidation of the alcohol
2 to the aldehyde followed by exposure to trimethyl
orthoformate led to the acetal
12. Reduction followed by exposure to acid gave 13, having four of the six rings of the natural product.
Oxidation to the enone followed by
conjugate addition installed the t-butyl group, unique to this
family of lactones. Exposure to base then converted the pendant alkyne to the
enol ether 14. Enolate formation followed by
α-hydroxylation with the Davis
oxaziridine and protection set the stage for oxidative cleavage of the enol
ether, leading to 15. A series of further transformations completed the
synthesis of ginkgolide C (3).
It is instructive to compare this approach to the previous syntheses of
ginkgolide B and A by Corey
(J. Am. Chem. Soc. 1988, 110, 649,
DOI: 10.1021/ja00210a083;
Tetrahedron Lett. 1988, 29, 3205, DOI:
10.1016/0040-4039(88)85122-0)
and of ginkgolide B by Crimmins
(J. Am. Chem. Soc. 1999, 121, 10249,
DOI: 10.1021/ja993013p;
2000, 122, 8453,
DOI: 10.1021/ja001747s).