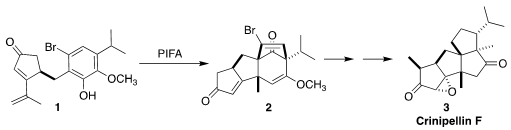
The crinipellins are the only tetraquinane natural products. The enone
crinipellins have anticancer activity. Hanfeng Ding of Zhejiang University
devised a general route to the crinipellins, illustrated by crinipellin F
(3), based on the oxidative cyclization of the
cyclopentenone 1 to the tricyclic enone 2
(J. PMID:23805407 Am. Chem. Soc. 2022, 144, 2495.
DOI: 10.1021/jacs.1c13370).
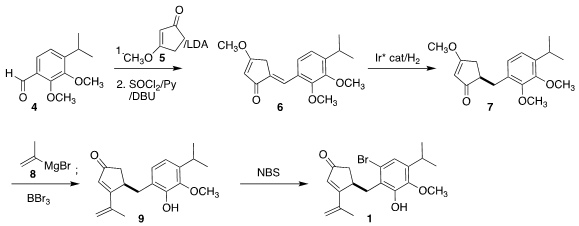
The starting point for the synthesis was the aldehyde 4. 1250997-29-5 In stock Addition of
the enolate derived from the vinylogous ester 5 followed by
dehydration led to the enone 6. Enantioselective
hydrogenation set the absolute
configuration of 7. 1-(4-Aminophenyl)ethan-1-ol Formula Addition of isopropenyl
Grignard 8 followed by
dehydration with BBr3 was accompanied by demethylation, leading to
the phenol 9. Although 9 participated efficiently in the oxidative
cyclization, the diastereoselectivity was improved significantly with the
derived bromide 1.
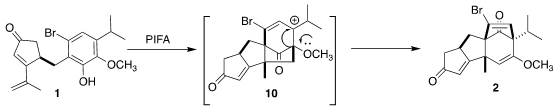
Exposure of the phenol 1 to
phenyliodine(III) bis-(trifluoroacetate)
and NaHCO3 at 0°C led smoothly to the tetracyclic bromide 2
and its easily separated diastereomer. The cyclization apparently proceeds by
way of the intermediate carbocation 10. With DFT calculations, Hujun Xie of Zhejiang Gongshang University assisted in the development of the cyclization.
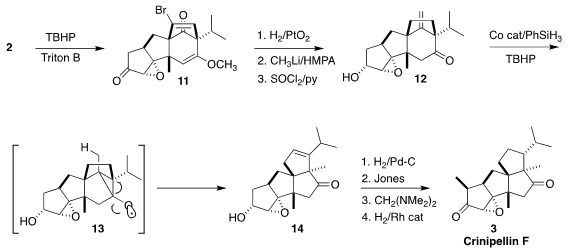
Addition of t-butyl hydroperoxide converted 2 to the solid
epoxide 11, the structure of which was confirmed by x-ray analysis of a
derivative. Hydrogenation over PtO2
reduced the ketone to the
alcohol, and removed the bromide and one of the alkenes. Addition of CH3Li
was followed by acidic workup to hydrolyze the enol ether. Dehydration then
delivered the alkene 12. Radical reorganization of 12 proceeded
via the anticipated Beckwith-Dowd rearrangement, leading to 14 by way of
the alkoxy radical 13. Hydrogenation followed by oxidation, methylenation
and reduction then completed the synthesis of crinipellin F (3).
Proceeding from these intermediates, the authors also prepared crinipellins
A, B, C, D and E, in the process revising the structure of crinipellin D. It is
striking that the accompanying experimental procedure describes the preparation
of 1.9 grams of 14.