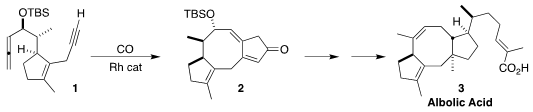
Albolic acid (3) and ceroplastol II, the corresponding primary alcohol, are
sesterterpenoids isolated from the wax secretion of Ceroplastes albolineatus, a
scale insect. Chuang-Chuang Li of the Southern University of Science and
Technology devised a route to 3 based on the Rh-catalyzed
Pauson-Khand
cyclization of the allene 1 to the tricyclic enone 2
(J. Am. Chem. Soc. 2,3-Dichloro-5-fluoropyridine site 2022, 144, 10162.
DOI: 10.1021/jacs.2c04633). PMID:35126464
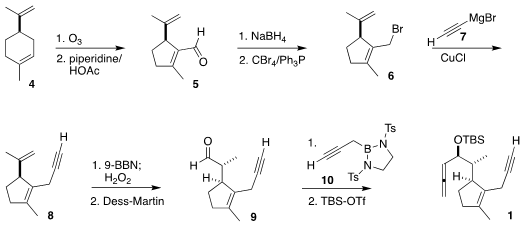
The synthesis started with the inexpensive limonene 4. 4-(Diethylphosphinyl)benzenamine Formula
Ozonolysis followed by
aldol condensation led to
5, that was reduced, then carried on to the
allylic
bromide 6. Coupling with ethynyl magnesium bromide 7 led to the volatile
alkyne 8. Diastereoselective hydroboration led after oxidation to the aldehyde
9. The
addition of the propargyl borane 10 was also highly diastereoselective,
completing the assembly of the yne-allene substrate 1.
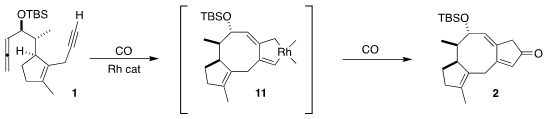
The Pauson-Khand cyclization probably proceeds via initial complexation with
the alkyne, followed by addition to the distal end of the allene, to give 11.
Carbonylation would then give 2. In the course of this work, the authors
demonstrated a high degree of functional group tolerance for this ring
construction strategy.
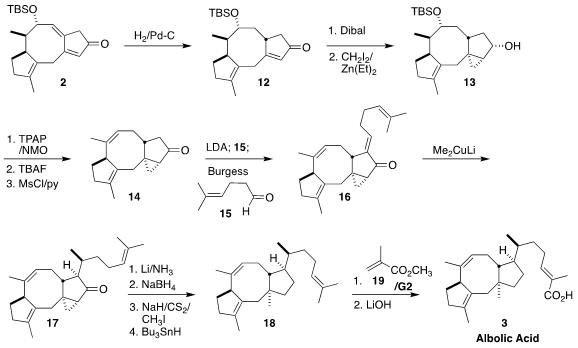
The conformation of the medium ring directed the hydrogenation of 2, to give
the enone 12. Conjugate addition to the congested system failed, so the
requisite angular methyl group was introduced by reduction to the allylic
alcohol followed by Simmons-Smith cyclopropanation, leading to
13. Oxidation followed by deprotection and dehydration led to the ketone
14. Aldol
condensation with the aldehyde 15 gave, after dehydration, the expected more
stable E-enone 16. Conjugate addition proceeded across the more open face of the
enone, establishing the sidechain stereocenter of the ketone 17. Dissolving
metal reduction followed by deoxygenation completed the assembly of the triene
18.
It would have been possible to achieve selective oxidation of the sidechain
alkene of 18. It was more expedient to effect
cross metathesis with methyl
methacrylate (19), leading, after saponification, to albolic acid (3).