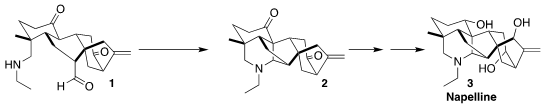
Napelline (3) was isolated from Aconitum kusnezoffii, a widely-cultivated
herbaceous perennial that, despite its toxicity, has long been used in
traditional medicine. Xiangbo Zhao and Dawei Ma of SIOC assembled the hexacyclic
ring system of 3 by the intramolecular
Mannich cyclization of 1 to
2
(J. Am. 644970-85-4 Data Sheet Chem. PMID:23577779 Soc. 2022, 144, 15355.
DOI: 10.1021/jacs.2c06738).
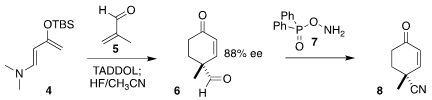
The convergent assembly of the diketone 1 began with the TADDOL-catalyzed
addition of methacrolein 5 to the diene 4, following the procedure of Rawal, to
give, after hydrolysis, the aldehyde 6. 5-Chloro-3-methylisoindolin-1-one web Warming with DPPH 7 converted the
aldehyde 6 to the nitrile 8.
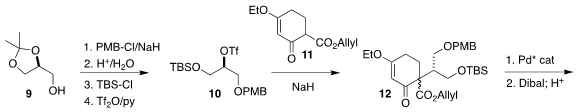
The preparation of the other half of 1 began with the
acetonide 9. Benzyl
ether formation and deprotection followed by selective
monosilylation led to the
secondary triflate 10, that was stabilized by the flanking electron-withdrawing
groups. Alkylation of 11 with 10 led to 12, that was rearranged with absolute
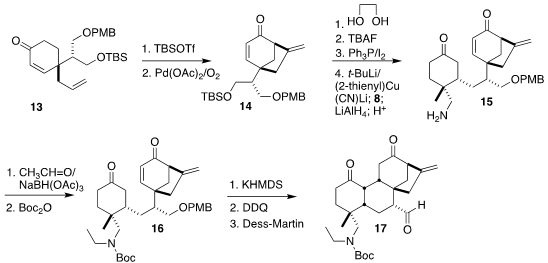
stereocontrol and then reduced and hydrolyzed, leading to the enone 13. Pd-mediated oxidative cyclization of the derived silyl enol ether gave
14.
Ketalization followed by iodide formation set the stage for transmetalation and
conjugate addition to 8. The product enolate was treated directly with
LiAlH4,
allowing selective reduction to the amine 15.
Reductive ethylation followed by
protection gave 16, that on deprotonation underwent intramolecular Michael
addition. Deprotection and oxidation completed the preparation of the aldehyde
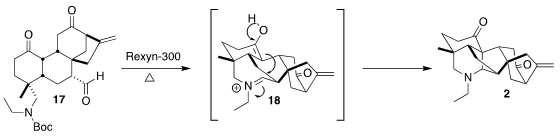
17.
Heat removed the Boc protecting group, so the key intramolecular Mannich
cyclization could proceed. This required the formation of the seven-membered
iminium salt 18, and so was reluctant. The use of the ion exchange resin
Rexyn-300, that has both strong base and strong acid sites, proved effective.
The cyclization of 17 to the hexacyclic 2 is a remarkable increase in molecular
complexity.
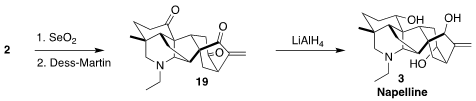
With 2 in hand, allylic oxygenation led to the triketone 19. Global reduction then completed the synthesis of napelline
(3).