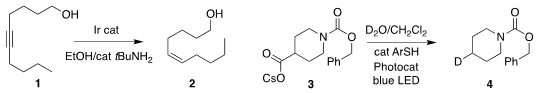
Zheng Huang of the Shanghai Institute of Organic Chemistry showed that with
an Ir catalyst, ethanol could serve as a reducing medium for the
conversion of
the alkyne 1 to the Z-alkene 2
(J. Am. Chem. Soc. 2021, 143, 4824.
DOI: 10.1021/jacs.1c01472).
Jin Xie, Weipeng Li and Chengjian Zhu of Nanjing University devised conditions for
the decarboxylative
deuteration of the cesium carboxylate 3 to 4
(Chem. Sci. 2021, 12, 5505.
DOI: 10.1039/D1SC00528F).
Jian Xu and Qi Wu of Zhejiang University reported an enzymatic system for the same transformation
(Nature Commun. 2021, 12, 3983.
DOI: 10.1038/s41467-021-24259-6). PMID:24381199
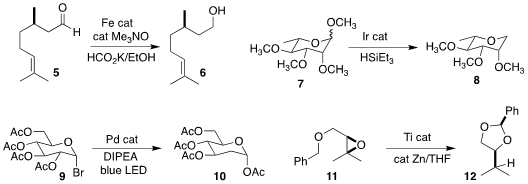
Jean-Luc Renaud of Normandie Université established that an iron catalyst could
effect the reduction of the unsaturated aldehyde 5 to the unsaturated alcohol 6
(Tetrahedron 2021, 90, 132187.
DOI: 10.1016/j.tet.2021.132187).
Nathan D. Schley of Vanderbilt University used an Ir catalyst to effect the selective
deoxygenation of the methyl glycoside
7 to the tetrahydropyran 8
(Chem. Buy5-Bromo-3-fluoropyridine-2-carbaldehyde Buy1639-66-3 Commun. 2021, 57, 5953.
DOI: 10.1039/D1CC00496D).
Ming-Yu Ngai of the State University of New York, Stony Brook used a Pd catalyst to accomplish
the selective 2-deoxygenation of the glucosyl bromide 9, leading to 10
(J. Am. Chem. Soc. 2021, 143, 1728.
DOI: 10.1021/jacs.0c11209).
Andreas Gansäuer of the Universität Bonn used a titanocene catalyst
to convert the epoxide 11 to the benzylidene acetal 12
(Angew. Chem. Int. Ed. 2021, 60, 5482.
DOI: 10.1002/anie.202013561).
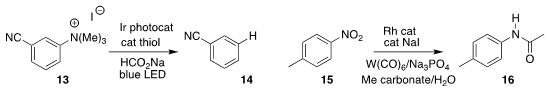
Nathan T. Jui of Emory University found conditions for the reduction of the
quaternary aryl ammonium salt 13 to the arene 14
(J. Am. Chem. Soc. 2021, 143, 8987.
DOI: 10.1021/jacs.1c04427).
Xinxin Qi of Zhejian Sci-Tech University and Xiao-Feng Wu of the Dalian Institute of Chemical Physics
reduced the nitroaromatic
15 in the presence of methyl carbonate, leading to the acetamide 16
(Chem. Commun. 2021, 57, 1955.
DOI: 10.1039/D1CC00047K).
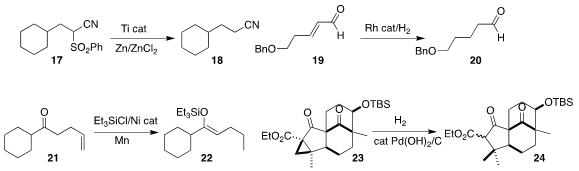
Jan Streuff of the Albert-Ludwigs-Universität Freiburg established that a titanocene
catalyst was also effective for the desulfonylation of 17, leading to the nitrile 18
(Chem. Eur. J. 2021, 27, 6178.
DOI: 10.1002/chem.202005400).
Jack R. Norton of Columbia University showed that with a Rh catalyst, the unsaturated aldehyde 19 could be
hydrogenated to the saturated aldehyde 20
(J. Am. Chem. Soc. 2021, 143, 9657.
DOI: 10.1021/jacs.1c04683).
Franziska Schoenebeck of RWTH Aachen used a Ni catalyst to mediate the migration
of the distal alkene of the ketone 21, leading to the
silyl enol ether 22
(J. Am. Chem. Soc. 2021, 143, 8375.
DOI: 10.1021/jacs.1c01797).
Shaomin Fu and Bo Liu of Sichuan University observed that hydrogenation could
open the activated cyclopropane of 23, to give 24
(Org. Lett. 2021, 23, 290.
DOI: 10.1021/acs.orglett.0c03748).
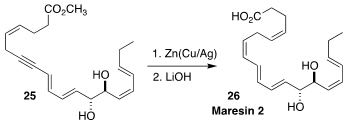
Maresin 2 (26), isolated from human macrophages, showed strong antiinflammatory
activity. Narihito Ogawa of Meiji University found that the reduction of the
alkyne 25 to the Z-alkene, often difficult with such systems of extended
conjugation, could be effected smoothly with activated zinc in methanol
(Synlett 2021, 32, 295.
DOI: 10.1055/s-0040-1705959).