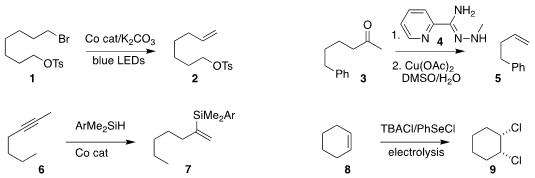
Daniele Leonori, now at RWTH Aachen University, employed visible light
to promote the Co-catalyzed elimination of the primary bromide 1
to the alkene 2
(J. 2091009-80-0 site Am. PMID:24211511 Chem. Soc. 2021, 143, 14806.
DOI: 10.1021/jacs.1c06768).
Yan Xu and Guangbin Dong of the University
of Chicago used the hydrazonamide 4 to mediate the deacylative conversion of the
ketone 3 to the alkene 5
(J. Am. Chem. Formula of 2-Bromo-5-(difluoromethyl)pyrazine Soc. 2021, 143, 20042.
DOI: 10.1021/jacs.1c09587).
Jung-Woo Park of KAIST established conditions for converting the internal alkyne 6 to the branched terminal
alkenyl silane 7
(ACS Catal. 2021, 11, 12777.
DOI: 10.1021/acscatal.1c03769).
Gerhard Hilt of the Universität Oldenburg devised electrolytic conditions for the conversion of
cyclohexene 8 to the cis 1,2-dichlorocyclohexane 9
(Chem. Eur. J. 2021, 27, 17341.
DOI: 10.1002/chem.202103316).
Masahiro Noji and Toshikatsu Takanami of Meiji Pharmaceutical University
optimized the rearrangement of the epoxide 10 to the allylic alcohol 11
(Chem. Commun. 2021, 57, 7104.
DOI: 10.1039/D1CC02840E).
Fabio Julía, also of the University of Manchester, and
Professor Leonori effected the conversion of the secondary iodide 12 to the
primary amine 13
(Nature Catal. 2021, 4, 623.
DOI: 10.1038/s41929-021-00652-8).
Tao Wang and Junkai Fu of Jiangxi
Normal University used 15 to selectively
demethylate the tertiary amine
14, leading to the amide 16
(Synlett 2021, 32, 1642.
DOI: 10.1055/a-1517-5895).
Kevin Lam of the University of
Greenwich prepared the isonitrile
18 by electrolytic oxidation of the
aminotetrazole 17
(Org. Lett. 2021, 23, 9371.
DOI: 10.1021/acs.orglett.1c03475).
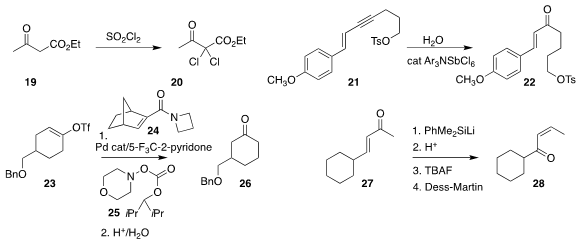
Qiang Tang of Chongqing Medical University found that sulfuryl chloride
smoothly converted the β-keto ester 19 to the
2,2-dichloro ketone
20
(Tetrahedron Lett. 2021, 81, 153335.
DOI: 10.1016/j.tetlet.2021.153335).
John E. Moses of Cold Spring Harbor
Laboratory used a catalytic aminium cation radical to
hydrate the eneyne 21
to
the enone 22
(Chem. Commun. 2021, 57, 6991.
DOI: 10.1039/D1CC02257A).
Professor Dong used a modified
Catellani protocol with the norbornene amide 24 and the N-acyloxy morpholine
25
to convert the enol triflate 23
to the ketone
26, a net 1,2-carbonyl
transposition
(Science 2021, 374, 734.
DOI: 10.1126/science.abl7854).
D. Srinivasa Reddy of CSIR-Indian
Institute of Integrative Medicine showed that 1,3-transposition of the E-enone
27 led cleanly to the Z-enone 28
(Org. Lett. 2021, 23, 6642.
DOI: 10.1021/acs.orglett.1c02173).
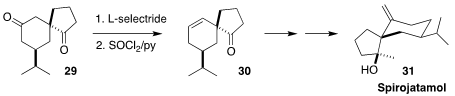
The sesquiterpene spirojatamol (31) was isolated from the roots of Nardostachys
jatamansi, a flowering plant of the valerian family that grows in the Himalayas.
In the course of a synthesis of 31, Shao-Hua Wang of Lanzhou University achieved
remarkable regioselectivity in the conversion of the ketone 29 to the alkene 30
(Tetrahedron Lett. 2021, 85, 153291.
DOI: 10.1016/j.tetlet.2021.153291).