A. PMID:28739548 Fmoc-Phe-OH Chemscene Venkat Narsaiah of CSIR-Indian Institute of Technology, Hyderabad
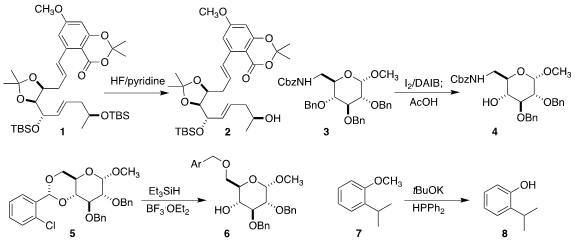
selectively removed
one of the silyl ethers of 1, to give 2
(Tetrahedron Lett. 2021, 82, 153410.
DOI: 10.1016/j.tetlet.2021.153410).
Andrés G. Santana of the Instituto de Productos Naturales y
Agrobiología del CSIC used the proximity of the adjacent carbamate to direct the
oxidative removal of one of the three
benzyl ethers of 3, leading to 4
(J. Org. Chem. 2021, 86, 16736.
DOI: 10.1021/acs.joc.1c01977).
Anikó Borbás of the University of Debrecen developed complementary protocols
for the reduction of 5 to either regioisomer of 6
(J. Org. Chem. 2021, 86, 12973.
DOI: 10.1021/acs.joc.1c01667).
Fangfang Li of Central South University and Tao Li
and Wanxiang Zhao of Hunan University used diphenylphosphine to
demethylate the
aryl ether 7 to the phenol 8
(Org. Biomol. 2422999-74-2 web Chem. 2021, 19, 7633.
DOI: 10.1039/D1OB01286J).
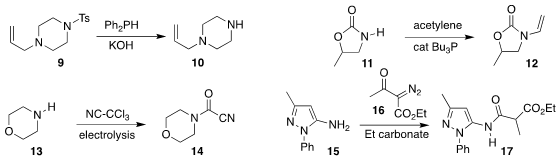
Guang Yang of Nankai University also used diphenylphosphine to convert
the
sulfonamide 9 to the free amine 10
(Org. Chem. Front. 2021, 8, 6182.
DOI: 10.1039/D1QO01190A).
Thomas Schaub of BASF showed that tributylphospine catalyzed the addition of the
urethane 11 to acetylene, leading to the N-vinylated product 12
(J. Org. Chem. 2021, 86, 13041.
DOI: 10.1021/acs.joc.1c01807).
Tobias Ritter of the Max-Planck-Institut für Kohlenforschung
developed an alternative vinylating reagent
(J. Am. Chem. Soc. 2021, 143, 12992.
DOI: 10.1021/jacs.1c06632).
Yoshio Hisaeda and Hisashi Shimakoshi used electrolysis to convert the
amine 13 to the cyanoformamide 14
(J. Org. Chem. 2021, 86, 16134.
DOI: 10.1021/acs.joc.1c00837).
Yan-Ping Zhu of Yantai University coupled the amine 15 with the α-diazo β-keto ester
16, leading via
Wolff rearrangement to the malonylated product 17
(J. Org. Chem. 2021, 86, 17471.
DOI: 10.1021/acs.joc.1c02165).
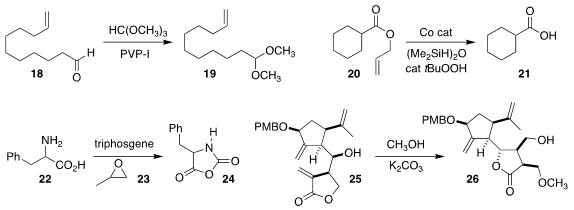
Jiangmeng Ren and Bu-Bing Zeng of the East China University of Science and
Technology showed that povidone-iodine was an effective catalyst for converting
the aldehyde 18
to the acetal 19
(Tetrahedron 2021, 92, 132250.
DOI: 10.1016/j.tet.2021.132250).
Nan Li, Meng Deng and Baoming Ji of Luoyang Normal University used a Co catalyst to prepare
the carboxylic acid 21 by the deprotection of the allyl ester 20
(Org. Lett. 2021, 23, 8460.
DOI: 10.1021/acs.orglett.1c03185).
Hua Lu of Peking University showed that in the presence of propylene oxide 23,
the amino acid 22 could be converted into the N-carboxyanhydride
24
(Nature Commun. 2021, 12, 5810.
DOI: 10.1038/s41467-021-25689-y).
Toyonobu Usuki of Sophia University used methanol to protect one of the
three alkenes of 25, as the rearranged lactone 26
(Org. Biomol. Chem. 2021, 19, 6038.
DOI: 10.1039/D1OB00657F).
The cyclooctanoid sesquiterpene jujuyane (29) was isolated from the cactus
Stevia jujuyensis of Argentina. In the course of a synthesis of 29, Hee-Yoon Lee
of KAIST protected one of the two alkenes of 27 as the bromo ether 28
(Org. Lett. 2021, 23, 4651.
DOI: 10.1021/acs.orglett.1c01391).