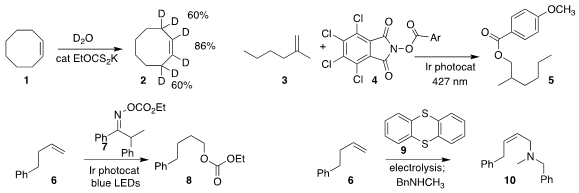
Qiang Liu and Yibiao Li of Wuyi University developed a simple protocol for
exchanging deuterium into the alkene 1, leading to 2
(Org. Lett. 2021, 23, 7412.
DOI: 10.1021/acs.orglett.1c02600).
Joseph M. Ready of the UT Southwestern Medical Center found that under
visible irradiation, the activated ester 4 could be added to the alkene
3, leading to the anti-Markovnikov ester 5
(ACS Catal. 2021, 11, 13714.
DOI: 10.1021/acscatal.1c03969).
In a parallel investigation, Bing Han of Lanzhou University assembled the anti-Markovnikov
carbonate 8 by photochemically-promoted
combination of the alkene 6 with the oxime carbonate 7
(Angew. Chem. Int. Ed. PMID:24238415 2021, 60, 21997.
DOI: 10.1002/anie.202107118).
Zachary K. Wickens of the University of Wisconsin showed that via electrolyic
oxidation of thianthracene 9, the alkene 6 could be converted to the
Z-allylic amine 10
(J. Am. Chem. Soc. Buy3-Fluoro-L-tyrosine 2021, 143, 21503.
DOI: 10.1021/jacs.1c11763).
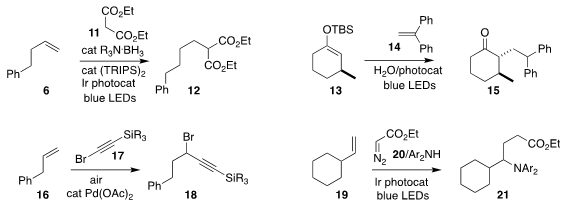
Ignacio Funes-Ardoiz of the Universidad de La Rioja and Juntao Ye of Shanghai
Jiao Tong University prepared the
alkylated malonate 12 by the
photochemically-promoted addition of diethyl malonate 11 to the alkene 6
(J. Am. Chem. 6-Bromopyrazin-2-amine site Soc. 2021, 143, 11251.
DOI: 10.1021/jacs.1c05852).
Yasuhiro Yamashita and Shū Kobayashi of the
University of Tokyo also used visible light to effect the coupling of the silyl
enol ether 13 with the alkene 14, leading to the
alkylated cyclohexanone 15
(Org. Lett. 2021, 23, 5693.
DOI: 10.1021/acs.orglett.1c01824).
Yusuke Ano of Osaka University used a Pd catalyst to mediate the 1,1-addition of the bromoalkyne
17 to the alkene 16, to give 18
(Chem. Sci. 2021, 12, 12326.
DOI: 10.1039/D1SC02873A).
Chao Yang and Wujiong Xia of the Harbin Institute
of Technology developed the photochemically-promoted addition of ethyl diazoacetate 20
and a diaryl amine to the alkene 19, leading to the γ-aminoester 21
(Org. Lett. 2021, 23, 6278.
DOI: 10.1021/acs.orglett.1c02071).
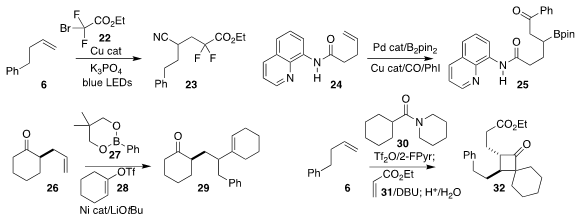
Under the photochemical conditions devised by Chuan-Ming Jin of Hubei Normal
University and Ji-Chang Xiao of the Shanghai Institute of Organic Chemistry,
cyanide was generated in situ, allowing the assembly of the cyanoester 23 by the
addition of the bromoacetate 22 to the alkene 6
(Chem. Commun. 2021, 57, 2649.
DOI: 10.1039/D1CC00160D).
Xiao-Feng Wu of the Dalian Institute of Chemical Physics established the directed Pd-catalyzed
carbonylation of the alkene 24, leading to the β-boryl ketone 25
(Chem. Sci. 2021, 12, 10341.
DOI: 10.1039/D1SC02785A).
Ramesh Giri of Pennsylvania State University assembled the ketone 29 by Ni-catalyzed
addition of the enol triflate 28 and the aryl borate 27 to the alkene 26
(Angew. Chem. Int. Ed. 2021, 60, 19092.
DOI: 10.1002/anie.202104871).
Alain de Mesmaeker of Syngenta showed that the intermediate enamine from the addition of the
dialkyl amide 30 to the alkene 6 could be coupled with ethyl acrylate 31, to give the
cyclobutanone 32
(Helv. Chim. Acta 2021, 104, e2100022.
DOI: 10.1002/hlca.202100022).
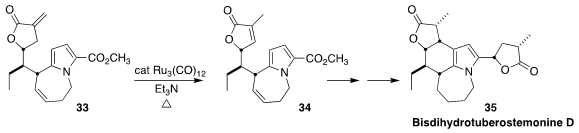
The alkaloid bisdihydrotuberostemonine D (35) was isolated from the roots of
the Chinese vine Stemona tuberosa. En route to 35, Yu-Rong Yang of the Kunming
Institute of Botany demonstrated that the exo methylene of 33 could
be
isomerized to the more stable endocyclic alkene 34 in the presence of the internal alkene
(J. Am. Chem. Soc. 2021, 143, 20622.
DOI: 10.1021/jacs.1c11265).