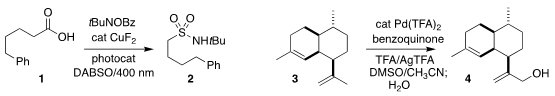
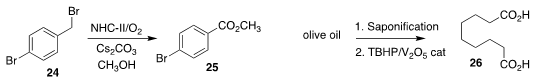Oleg V. Larionov of the University of Texas at San Antonio used an acridine
photocatalyst to mediate the decarboxylative conversion of the carboxylic acid
1
to the sulfonamide 2
(Chem. 210539-05-2 Chemical name BuyBis(triphenylphosphine)dichloronickel Sci. 2021, 12, 6429.
DOI: 10.1039/D1SC01389K).
Zacharias Amara of Cnam, HESAM Université and Janine Cossy of ESPCI Paris
optimized the selective oxidation of amorphadiene 3 to the
allylic alcohol 4
(J. Org. Chem. 2021, 86, 7603.
DOI: 10.1021/acs.joc.1c00653).
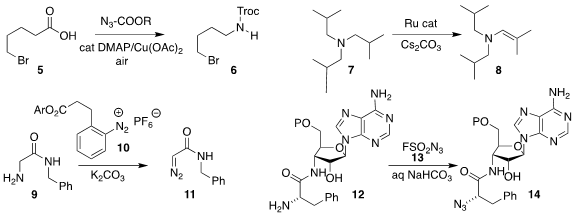
Hongjian Lu of Nanjing University devised the oxidative decarboxylation of
the carboxylic acid 5 to the
protected amine 6
(Angew. PMID:24856309 Chem. Int. Ed. 2021, 60, 1845.
DOI: 10.1002/anie.202010974).
Bill Morandi of ETH Zürich used a Ru catalyst to oxidize the tertiary
amine 7 to the
enamine 8
(Angew. Chem. Int. Ed. 2021, 60, 7290.
DOI: 10.1002/anie.202015837).
Ho-Hsuan Chou of the National Cheng Kung University developed the reagent 10 to directly
convert the α-amino amide 9 to the α-diazo amide 11
(Chem. Commun. 2021, 57, 4839.
DOI: 10.1039/D1CC01285A).
Ronald Micura of the University of Innsbruck showed that the reagent 13 could convert RNA
12 bearing an α-amino amide to RNA 14 bearing an α-azido amide
(Angew. Chem. Int. Ed. 2021, 60, 6970.
DOI: 10.1002/anie.202015034).
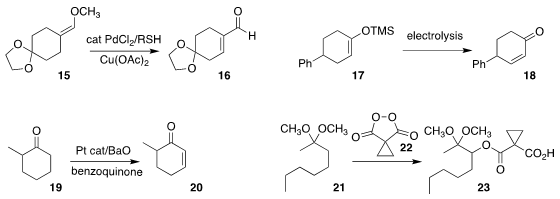
Yunhai Tao of Yunnan University found that octadecanethiol mediated the
oxidation of the enol ether 15 to the
unsaturated aldehyde 16
(J. Org. Chem. 2021, 86, 5463.
DOI: 10.1021/acs.joc.0c02987).
Phil S. Baran of Scripps/La Jolla showed that a silyl enol ether 17 could
be converted by electrolysis to the α,β-unsaturated ketone 18
(Nature Chem. 2021, 13, 367.
DOI: 10.1038/s41557-021-00640-2).
Guangbin Dong of the University of Chicago
observed high regioselectivity in the direct oxidation of the ketone 19 to the
α,β-unsaturated ketone 20
(Angew. Chem. Int. Ed. 2021, 60, 7956.
DOI: 10.1002/anie.202013628).
Alexander O. Terent’ev of the N. D. Zelinsky Institute of Organic Chemistry also achieved high
regioselectivity in the coupling of the ketal 21 with the reagent 22, leading to
the ester 23
(Org. Chem. Front. 2021, 8, 3091.
DOI: 10.1039/D1QO00494H).
Ning Jiao of Peking University showed that a catalytic N-heterocyclic carbene
could mediate the oxidation of the benzylic bromide 24 to the
ester 25
(Angew. Chem. Int. Ed. 2021, 60, 2140.
DOI: 10.1002/anie.202011039).
Sushil K. Maurya of CSIR-Institute of Himalayan
Bioresource Technology used a reusable vanadium catalyst to oxidize olive oil
and also non-edible vegetable oils to azelaic acid (26)
(Chem. Commun. 2021, 57, 5430.
DOI: 10.1039/D1CC01742J).
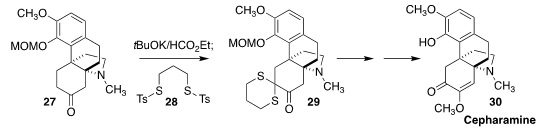
The alkaloid cepharamine (30) was isolated from the Korean succulent Stephania
cepharantha. In the course of a synthesis of 30, Jieping Zhu of the Ecole
Polytechnique Fédérale de Lausanne observed high regioselectivity in the
assembly of the thioketal 29 by the coupling of the ketone 27 with the reagent 28
(Nature Commun. 2021, 12, 36.
DOI: 10.1038/s41467-020-20274-1).