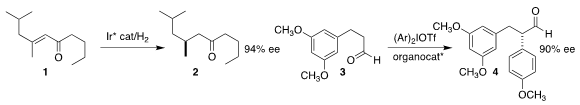
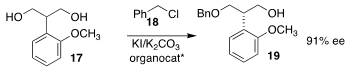Pher G. Andersson of Stockholm University achieved high ee in the
hydrogenation of the enone
1 to the ketone 2
(Org. 6-Bromo-2,3-dihydrobenzofuran manufacturer Fmoc-Phe(CF2PO3)-OH site Lett. PMID:23937941 2021, 23, 242.
DOI: 10.1021/acs.orglett.0c04012).
Shinji Tanimori of Osaka Prefecture University used the MacMillan catalyst to
arylate
the aldehyde 3, leading to 4
(Synlett 2021, 32, 693.
DOI: 10.1055/a-1303-9935).
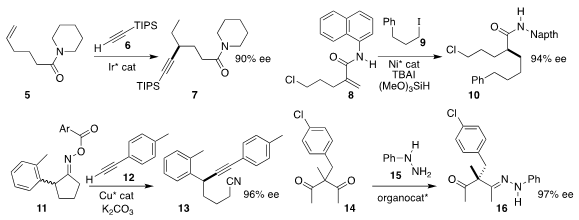
Bi-Jie Li of Tsinghua University prepared the amide 7 by the migratory
hydroalkynylation of the terminal alkene 4 with the alkyne 6
(Tetrahedron Lett. 2021, 73, 153108.
DOI: 10.1016/j.tetlet.2021.153108).
Wei Shu of the Southern University of Science and Technology
set the α stereocenter of the amide 10 by the conjugate addition of the iodide
9 to the α-methylene amide 8
(Angew. Chem. Int. Ed. 2021, 60, 1599.
DOI: 10.1002/anie.202011339).
Wen-Juan Hao, Shu-Jiang Tu and Bo Jiang of Jiangsu Normal University assembled the nitrile 13 by
the fragmentative coupling of the oxime ester 11 with the alkyne 12
(ACS Catal. 2021, 11, 6010.
DOI: 10.1021/acscatal.1c00842).
Yu Lan of Chongqing University, Zhihui Shao of Yunnan University and Yu Zhao of the National
University of Singapore used a chiral phosphoric acid to direct the enantioselective
formation of the hydrazone 16
from the prochiral diketone 14 and phenylhydrazine 15
(J. Am. Chem. Soc. 2021, 143, 4179.
DOI: 10.1021/jacs.1c01366).
Dennis G. Hall of the University of Alberta used a chiral hemiboronic acid to
enantioselectively protect the prochiral diol
17 with benzyl chloride (18), leading
to the alcohol 19
(J. Am. Chem. Soc. 2021, 143, 4162.
DOI: 10.1021/jacs.1c00759).
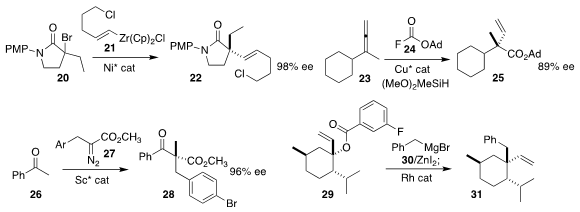
Gregory C. Fu of Caltech devised the enantioselective coupling of the racemic
α-bromo amide 20 with the
alkenylzirconium nucleophile
21, leading to the α-alkenyl amide 22
(Nature Chem. 2021, 13, 236.
DOI: 10.1038/s41557-020-00609-7).
Stephen L. Buchwald of MIT assembled the α-alkenyl ester 25 by coupling the commercial fluoroformate
24 with the allene 23
(J. Am. Chem. Soc. 2021, 143, 4935.
DOI: 10.1021/jacs.1c01880).
Xiaohua Liu and Xiaoming Feng of Sichuan University and Yun-Dong Wu of Peking University Shenzhen Graduate School
inserted the α-diazo ester 27 into the ketone 26, leading to the keto ester 28
(J. Am. Chem. Soc. 2021, 143, 2394.
DOI: 10.1021/jacs.0c12683).
P. Andrew Evans of Queen’s University observed predominantly inversion of absolute configuration in the
establishment of the cyclic quaternary center of 31 by the coupling of the
Grignard reagent 30 with the tertiary allylic ester 29
(Angew. Chem. Int. Ed. 2021, 60, 2987.
DOI: 10.1002/anie.202008071).
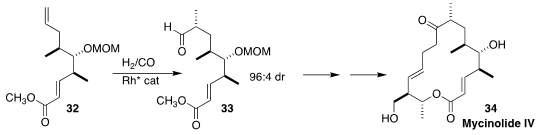
Mycinolide IV (34), isolated from Micromonospora griseorubida, was shown to
overcome erythromycin resistance in clinically isolated Staphylococcus aureus
strains. Alois Fürstner of the Max-Planck-Institut für Kohlenforschung set one
of the ternary stereocenters of 34 by the asymmetric
hydroformylation of the
alkene 32 to the aldehyde 33
(Angew. Chem. Int. Ed. 2021, 60, 7893.
DOI: 10.1002/anie.202016475).