Wesley J. 1-(3-Aminopropyl)azepan-2-one Order 2-Bromo-4,5-difluoropyridine Chemscene Moran of the University of Huddersfield cyclized
(Tetrahedron Lett. 2011, 52, 2605.
DOI: 10.1016/j.tetlet.2011.03.086)
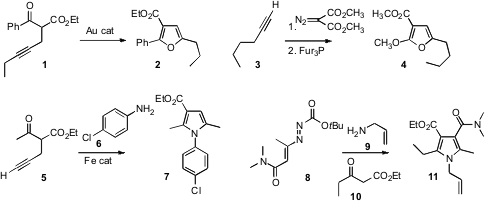
a propargylated ketone 1 with Au to give the
furan 2.
Shengming Ma of the Shanghai Institute of Organic Chemistry found
(Synlett 2011, 931.
DOI: 10.1055/s-0030-1259904)
that tri(2-furyl)phosphine catalyzed the rearrangement of cyclopropene diesters,
prepared by the addition of diazomalonate to alkynes, to the corresponding
alkoxy furan 4.
Xihe Bi and Qian Zhang of Northeast Normal University established
(Chem. PMID:35991869 Commun. 2011, 47, 809.
DOI: 10.1039/C0CC03802D)
that a simple Fe catalyst effected the condensation of 5 with 6
to give the pyrrole 7. Gianfranco Favi of the Università degli Studi di Urbino showed
(J. Org. Chem. 2011, 76, 2860.
DOI: 10.1021/jo200287k)
that the three-component coupling of 8 with an amine 9 and a keton
10 proceeded without catalyst to deliver the pyrrole 11.
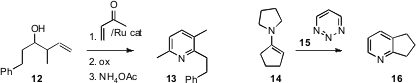
Timothy J. Donohoe of the University of Oxford homologated
(Org. Lett. 2011, 13, 1036.
DOI: 10.1021/ol103088r)
12 by cross metathesis with methyl vinyl ketone, to give, after
oxidation and condensation with NH4OAc, the substituted
pyridine
13. Dale L. Boger of Scripps/La Jolla condensed
(Org. Lett. 2011, 13, 2492.
DOI: 10.1021/ol2007428)
the unsubstitued 1,2,3-triazine 15 with an enamine 14 to
give 16. Shunsuke Chiba of Nanyang Technological University prepared
(J. Am. Chem. Soc. 2011, 133, 6411.
DOI: 10.1021/ja200879w)
the pyridine 19 by oxidizing the
cyclopropanol 17 in the presence of the alkenyl
azide 18. Limin Wang of the East China University of Science and Technology prepared
(Tetrahedron Lett. 2011, 52, 509.
DOI: 10.1016/j.tetlet.2010.11.102)
the 2-aminopyridine 23 by the
four-component coupling of the aldehyde 20
and the ketone 22 with NH4OAc and malononitrile 21.
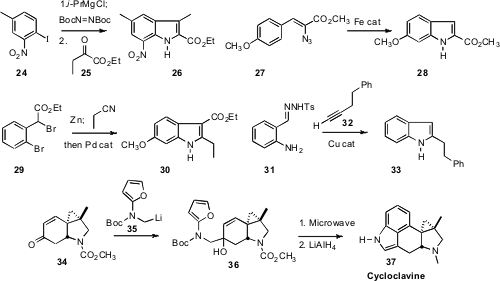
Taking advantage of the Knochel protocol for aryl Grignard formation,
Christopher J. Moody of the University of Nottingham combined
(Chem. Commun. 2011, 47, 788.
DOI: 10.1039/C0CC04306K)
the adduct from 24 with the ketone 25 to give the
indole 26.
Carsten Bolm of RWTH Aachen extended
(Org. Lett. 2011, 13, 2012.
DOI: 10.1021/ol2004066)
the Fe catalysis reported by Zheng to the azide 27 to give 28.
Sang-gi Lee of Ewha Womans University found
(Org. Lett. 2011, 13, 1350.
DOI: 10.1021/ol200045q)
that the Blaise adduct from the addition of 29 to the nitrile
could be cyclized to the indole 30. Jianbo Wang of Peking University applied
(Org. Lett. 2011, 13, 968.
DOI: 10.1021/ol103009n)
the allene synthesis that he had developed to the aniline 31, to give 33.
We (Tetrahedron 2011, 67, 7195.
DOI: 10.1016/j.tet.2011.06.040)
reported a systemization of the nine
fundamental strategies for indole synthesis. Of those strategies, the rarest is
the construction of both rings at once. That approach is elegantly illustrated
by the assembly of Cycloclavine 37 devised
(J. Am. Chem. Soc. 2011, 133, 7704.
DOI: 10.1021/ja2026882)
by Peter Wipf of the University of Pittsburgh.