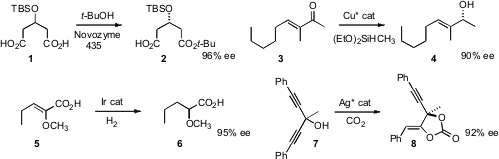
Computational analysis of the Novozyme 435 active site led
(Tetrahedron Lett. 2010, 51, 309.
DOI: 10.1016/j.tetlet.2009.11.008)
Liyan Dai and Hongwei Yu of Zhejiang University, Hangzhou to t-butanol for the enantioselective monoesterification of 1 to 2.
Bruce H. Decyl acrylate uses Lipshutz of the University of California, Santa Barbara devised
(J. Am. Oxetane-3-carboxylic acid uses Chem. Soc. 2010, 132, 7852.
DOI: 10.1021/ja102689e)
a Cu catalyst that mediated the enantioselective
1,2-reduction of α-branched
enones such as 3. Qi-Lin Zhou of Nankai University found
(J. Am. Chem. Soc. 2010, 132, 1172.
DOI: 10.1021/ja909810k)
that an α-alkoxy unsaturated acid 5 could be hydrogenated with high ee.
Tohru Yamada of Keio University desymmetrized
(J. Am. Chem. Soc. 2010, 132, 4072.
DOI: 10.1021/ja1007118)
the tertiary alcohol 7, delivering the enol
lactone 8.
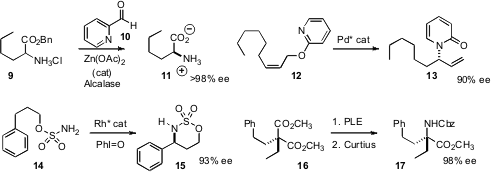
Zachary D. PMID:35670838 Aron of Indiana University established
(Org. Lett. 2010, 12, 1916.
DOI: 10.1021/ol100319b)
that the simple aldehyde 10 effected rapid racemization of the α-amino
ester 9. Running the epimerization in the presence of an enantioselective
esterase produced 11 high ee. Robert A. Batey of the University of Toronto devised
(Org. Lett. 2010, 12, 260.
DOI: 10.1021/ol9025759)
a Pd catalyst for the enantioselective rearrangement of 12 to 13.
In the course of a synthesis of Dapoxetine, Hyeon-Kyu Lee of the Korea
Research Institute of Chemical Technology showed
(J. Org. Chem. 2010, 75, 237.
DOI: 10.1021/jo902176s)
that the Rh*-mediated intramolecular C-H insertion of 14 to 15, as developed by
Du Bois, gave the opposite absolute configuration to that originally assigned.
To prepare α-quaternary amines, Thomas G. Back of the University of Calgary explored
(J. Org. Chem. 2010, 75, 1612.
DOI: 10.1021/jo902584r)
the selectivity of the PLE hydrolysis of esters such as 16.
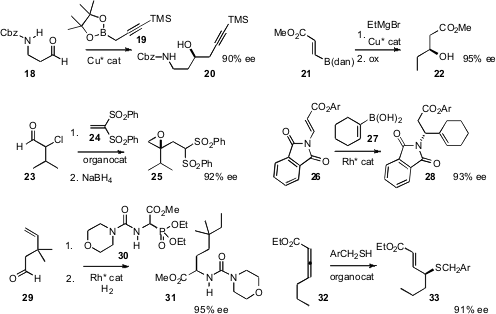
Daniel R. Fandrick and co-workers at Boehringer Ingelheim reported
(J. Am. Chem. Soc. 2010, 132, 7600.
DOI: 10.1021/ja103312x)
a general method for the catalytic enantioselective propargylation of aldehydes, including 18.
Dennis G. Hall of the University of Alberta devised
(J. Am. Chem. Soc. 2010, 132, 5544.
DOI: 10.1021/ja9104057)
a route to β-hydroxy esters such as 22, by enantioselective
conjugate addition to 21.
Alexandre Alexakis of the University of Geneva prepared
(Chem. Commun. 2010, 46, 4085.
DOI: 10.1039/C000326C)
disubstituted epoxides such as 25 by the conjugate addition of 23 to 24.
Takahiro Nishimura and Tamio Hayashi of Kyoto University, and Albert S. C. Chan
and of the Hong Kong Polytechnic University, prepared
(J. Am. Chem. Soc. 2010, 132, 464.
DOI: 10.1021/ja909642h)
the β-amino ester 28 by enantioselective conjugate addition to 26.
Jon C. Lorenz and co-workers, also at Boehringer Ingelheim, prepared
(J. Org. Chem. 2010, 75, 1155.
DOI: 10.1021/jo9022809)
the phosphonate 30. The product from the condensation of 30 with the
aldehyde 29 could be hydrogenated to 31 in high ee.
Gregory C. Fu of MIT showed
(J. Am. Chem. Soc. 2010, 132, 4568.
DOI: 10.1021/ja101251d)
that thiol addition to the allene ester 32 could be effected with high ee. Routes to high
ee α-vinyl thioethers
(J. Org. Chem. 2010, 75, 4615.
DOI: 10.1021/jo1006152),
α-halo acids
(J. Am. Chem. Soc. 2010, 132, 2860.
DOI: 10.1021/ja910281s)
and α-silyl esters
(J. Am. Chem. Soc. 2010, 132, 4510.
DOI: 10.1021/ja100833h)
have also been reported.