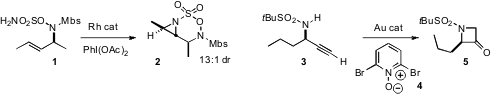Barry M. 3-Methoxy-1H-indole Chemical name Trost and Justin Du Bois of Stanford University described
(Org. PMID:23672196 Lett. 90396-00-2 manufacturer 2011, 13, 3336.
DOI: 10.1021/ol2010769)
the cyclization of 1 to the activated
aziridine 2. Liming Zhang
of the University of California, Santa Barbara rearranged
(Angew. Chem. Int. Ed. 2011, 50, 3236.
DOI: 10.1002/anie.201007624)
the propargylic amine 3 to the azetidinone
4, by N-H insertion of an intermediate Au carbene.
Xiao Zheng and Pei-Qiang Huang of Xiamen University effected
(J. Org. Chem. 2011, 76, 4952.
DOI: 10.1021/jo200600n)
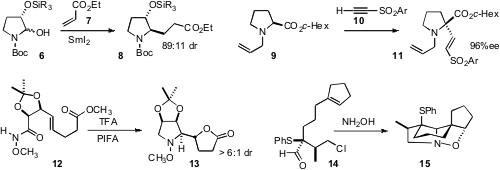
reductive coupling of 6 with 7 to deliver the ester
8. Eiji Tayama of Niigata University found
(Tetrahedron Lett. 2011, 52, 1819.
DOI: 10.1016/j.tetlet.2011.02.040)
that 9 could be alkenylated with 10 with substantial retention of absolute
configuration. Duncan J. Wardrop of the University of Illinois at Chicago, en
route to a synthesis of (-)-Swainsonine, observed
(Org. Lett. 2011, 13, 2376.
DOI: 10.1021/ol2006117)
high diastereocontrol in the cyclization of 12 to 13. Iain Coldham of the
University of Sheffield also observed
(J. Org. Chem. 2011, 76, 2360.
DOI: 10.1021/jo2000868)
substantial diastereoselection in the cyclization of
14 to 15.
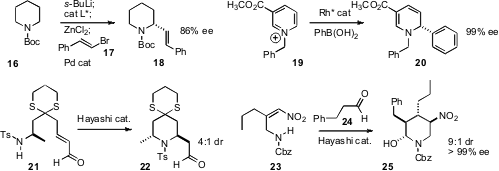
Robert E. Gawley of the University of Arkansas established
(Org. Lett. 2011, 13, 394.
DOI: 10.1021/ol102682r)
that exposure of metalated 16 to just 5 mol % of a chiral ligand was
sufficient to enable enantioselective coupling, to deliver 17. Christian Nadeau
of Merck Frosst effected
(J. Am. Chem. Soc. 2011, 133, 2878.
DOI: 10.1021/ja111540g)
enantioselective addition to the pyridinium salt 19 to give 20.
Jiyong Hong of Duke University observed
(Org. Lett. 2011, 13, 796.
DOI: 10.1021/ol103064f)
that enantiomerically pure 21 cyclized to
the cis diastereomer of 22. With the Hayashi catalyst, cyclization could be
driven toward the trans diastereomer, 22, enabling the synthesis of (+)-Myrtine.
Dawei Ma of the Shanghai Institute of Organic Chemistry found
(Org. Lett. 2011, 13, 1602.
DOI: 10.1021/ol200004s)
that the Hayashi catalyst also directed the relative and absolute
outcome in the addition of 24 to 23, to give the
piperidine 25.
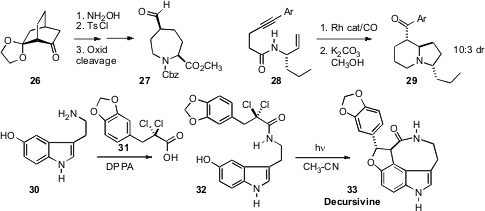
Donn G. Wishka of Pfizer/Groton devised
(J. Org. Chem. 2011, 76, 1937.
DOI: 10.1021/jo102475s)
a practical route to the cis-substituted azepane 27, by
Beckmann rearrangement of
the enantiomerically-pure 26 followed by reduction and oxidative cleavage.
Wen-Hua Chiou of the National Chung Hsing University formylated
(Chem. Comm. 2011, 47, 3562.
DOI: 10.1039/C0CC05646D)
28, to give, after cyclization, the
bicyclic ketone
29.
Mark Mascal of the University of California, Davis crafted
(Angew. Chem. Int. Ed. 2011, 50, 4445.
DOI: 10.1002/anie.201006423)
an elegant route to the antimalarial Decursivine
(33), based on the Witkop cyclization of 32.
Remarkably, Yanxing Jia of Peking University devised
(Angew. Chem. Int. Ed. 2011, 50, 4447.
DOI: 10.1002/anie.201100495)
a nearly identical synthesis.