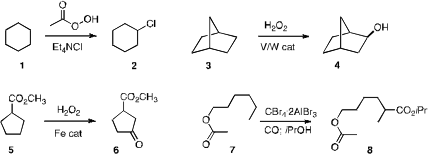
Christian R. Buy27194-74-7 Goldsmith of Auburn University developed
(Synlett 2010, 1377.
DOI: 10.1055/s-0029-1219832)
a method for radical chlorination of 1, using commercial peracetic
acid. PMID:23613863 Noritaka Mizuno of the University of Tokyo devised
(Nat. Chem. 2010, 2, 478.
DOI: 10.1038/nchem.648)
a bulky polyoxometalate that mediated the selective hydroxylation of the secondary
C-H bonds of 3. Christina White of the University of Illinois showed
(Science 2010, 327, 566.
DOI: 10.1126/science.1183602)
that Fe-mediated C-H oxidation is sensitive to the expected electronic effects, so
that 5 was selectively oxidized to 6. Irena S. Akhrem of the A. Buy1703768-74-4 N.
Nesmeyanov Institute of Organoelement Compounds established
(Tetrahedron Lett. 2010, 51, 259.
DOI: 10.1016/j.tetlet.2009.10.132)
that a C-H bond of 7 could be efficiently converted to a C-C bond.
Melanie S. Sanford of the University of Michigan extended
(Org. Lett. 2010, 12, 532.
DOI: 10.1021/ol902720d)
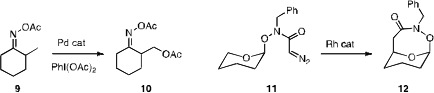
directed palladation to 9, effecting selective acetoxylation of the
methyl group. Herman O. Sintim of the University of Maryland observed
(Angew. Chem. Int. Ed. 2010, 49, 3964.
DOI: 10.1002/anie.201000160)
that the O-linked diazoamide 11 selectively cyclized to 12. The
corresponding C-linked diazoamide gave only five-membered ring formation.
Yasushi Obora and Yasutaka Ishii of Kansai University devised
(Org. Lett. 2010, 12, 1372.
DOI: 10.1021/ol100292g)
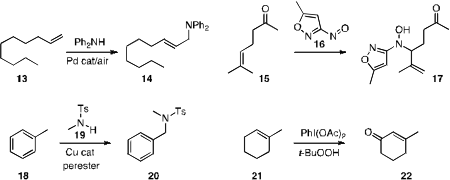
conditions for the selective allylic amination of 13. Marvin J. Miller
of the University of Notre Dame developed
(Tetrahedron Lett. 2010, 51, 328.
DOI: 10.1016/j.tetlet.2009.11.015)
the nitrosoisoxazole 16 for the allylic amination of 15.
David A. Powell of Merck Frosst established
(J. Org. Chem. 2010, 75, 2726.
DOI: 10.1021/jo100197r)
a protocol for the selective amination of the aromatic methyl group of 18.
Ying-Yeung Yeung of the National University of Singapore effected
(Org. Lett. 2010, 12, 2128.
DOI: 10.1021/ol100603q)
selective allylic oxidation of 21 with a
hypervalent iodine
reagent.
Gullapalli Kumaraswamy of the Indian Institute of Chemical Technology,
Hyderabad allylated
(J. Org. Chem. 2010, 75, 3916.
DOI: 10.1021/jo1005813)
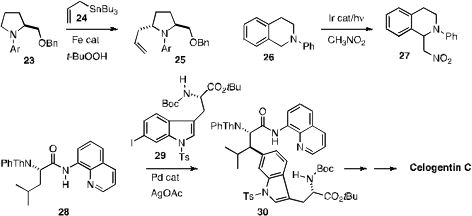
an amine 23 using commercial aqueous
t-BuOOH. Corey R. J. Stephenson of
Boston University used
(J. Am. Chem. Soc. 2010, 132, 1464.
DOI: 10.1021/ja909145y)
visible light to activate 26 for homologation to 27.
In the course of a synthesis of the bicyclic nonribosomal peptide Celogentin
C, isolated from the seeds of the plumed cockscomb Celosia argentea, Gong Chen
of Pennsylvania State University took advantage
(Angew. Chem. Int. Ed. 2010, 49, 958.
DOI: 10.1002/anie.200905134)
of Pd activation to effect specific coupling of the
iodoindole 29 with the leucine derivative 28. On a four-gram scale,
this coupling proceeded in 85% yield.