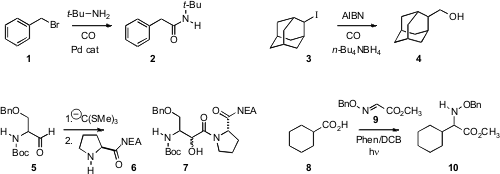
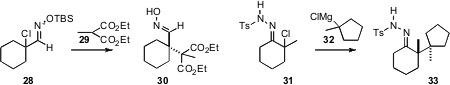Luigino Troisi of the University of Salento found
(Tetrahedron Lett. 2010, 51, 371.
DOI: 10.1016/j.tetlet.2009.11.023)
that a variety of primary and secondary amines could be coupled with a benzylic halide
1 under carbonylating conditions. Ilhyong Ryu of Osaka Prefecture University showed
(Org. Lett. 2010, 12, 1548.
DOI: 10.1021/ol1002847)
that under reducing conditions, an iodide 3 coupled with CO to give the primary alcohol.
Felicia A. Etzkorn of Virginia Tech observed
(Org. Lett. 2010, 12, 696.
DOI: 10.1021/ol9027013)
that under Hg hydrolysis conditions, the orthothioester derived from 5 coupled with 6 to give
7. Yasuharu Yoshimi of the University of Fukui and Minoru Hatanaka of Iwate Medical University devised
(Tetrahedron Lett. PMID:24189672 2010, 51, 2332.
DOI: 10.1016/j.tetlet.2010.02.112)
conditions for the decarboxylative addition of the acid 8 to 9 to give 10. Yong-Min Liang and
Xiaojun Yao of Lanzhou University and Chao-Jun Li of McGill University described
(J. Org. Chem. 2010, 75, 783.
DOI: 10.1021/jo902319h)
a related procedure with α-amino acids.
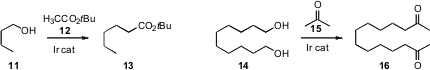
Yasutaka Ishii of Kansai University established
(J. Am. Triazabicyclodecene Chemical name 1339559-21-5 custom synthesis Chem. Soc. 2010, 132, 2536.
DOI: 10.1021/ja9106989)
that t-butyl acetate 12 was an effective partner for the Ir-mediated
oxidation-coupling-reduction of an alcohol 11. He used
(J. Org. Chem. 2010, 75, 1803.
DOI: 10.1021/jo9027165)
a similar protocol to condense acetone with the diol 14, to give the
long-chain diketone 16.
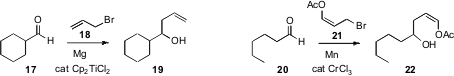
The formation of allylic Grignard reagents can be inefficient, as the excess
reactive halide tends to couple with the Grignard reagent as it forms. Brandon
L. Ashfeld of the University of Notre Dame found
(Tetrahedron Lett. 2010, 51, 2427.
DOI: 10.1016/j.tetlet.2010.02.144)
a simple solution to this problem, inclusion of a catalytic amount of the
inexpensive Cp2TiCl2 to mediate the addition of 18 to
17. Brian T. Connell of Texas A&M demonstrated
(J. Am. Chem. Soc. 2010, 132, 7826.
DOI: 10.1021/ja910057g)
that with Mn, 21 could be added to 20. The acetate 21 is thus an
easily prepared homoenolate equivalent. Note that although 21 is an E/Z
mixture, the product 22 is cleanly Z.
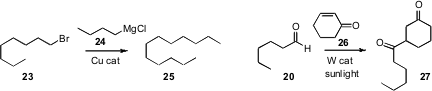
Gérard Cahiez of the Université de Paris 13 reported
(Synlett 2010, 299.
DOI: 10.1055/s-0029-1219222)
a detailed study of the Cu-catalyzed coupling of 24 with 23. Without supporting
ligands, slow addition (syringe pump, 1 h) of 24 to 23 assured clean formation
of 25. Manual slow addition (dropping funnel, 15 min) was not effective.
The preparatively-useful generation of formyl radicals from aldehydes has
long been desired. Maurizio Fagnoni of the University of Pavia found
(Chem. Commun. 2009, 7351.
DOI: 10.1039/B917732A)
that a tungstate catalyst under sunlight mediated the
conjugate addition of 20 to 26. Unsaturated esters were also efficient acceptors.
Stephen Caddick of University College London extended
(Chem. Commun. 2010, 46, 133.
DOI: 10.1039/B914563J)
this work to unsaturated sulfones and sulfonates. He found that addition
could be effected simply by combining the reactants on water in the presence of
air.
The construction of contiguous quaternary alkylated centers is particularly
challenging. Steven M. Weinreb of Pennsylvania State University showed
(Tetrahedron Lett. 2010, 51, 2032.
DOI: 10.1016/j.tetlet.2010.02.050)
that the protected chloro oxime 28 coupled smoothly with 29 to give
30. In a related development, Don M. Coltart of Duke University found
(J. Am. Chem. Soc. 2010, 132, 4546.
DOI: 10.1021/ja100932q)
that the tosylhydrazone 31 could be coupled with 32 to give 33.