(Taylor), Goniothalesdiol A (Xie/She), (-)-7-Deoxyloganin (Lupton), (-)-Apicularen
A (Uenishi)L-783, 277 (Banwell)
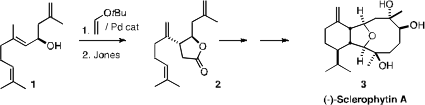
In the course of a synthesis of (-)-Sclerophytin A (3), James P. Morken of Boston College showed
(J. Am. Chem. Soc. 2010, 132, 16380.
DOI: 10.1021/ja108185z)
that Oshima-Utimoto conditions transformed 1 into 2 with high stereo- and regiocontrol.
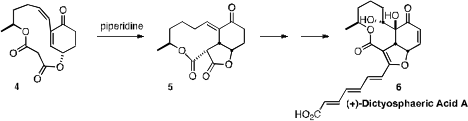
En route to (+)-Dictyosphaeric Acid (6), Richard J. K. PMID:23892407 (S)-(+)-Norepinephrine L-bitartrate Price Taylor of the University of York found
(Angew. Chem. Int. Lumisterol 3 (>90%) site Ed. 2010, 49, 5574.
DOI: 10.1002/anie.201002416)
that the intramolecular Michael cyclization of
4 proceeded smooothly to give 5.
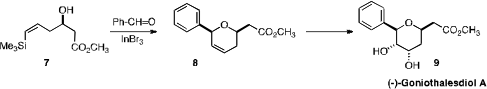
Xingang Xie and Xuegong She of Lanzhou University devised
(Synlett 2010, 2283.
DOI: 10.1055/s-0030-1258016)
the In-mediated cyclization of 7 with benzaldehyde, to effect an elegant
synthesis of Goniothalesdiol A (9).
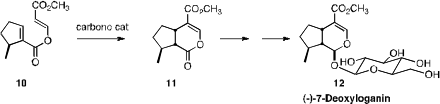
The carbene-mediated cyclization of 10 to 11 developed
(Org. Lett. 2010, 12, 4836.
DOI: 10.1021/ol101983h)
by David W. Lupton of Monash University set the stage for the synthesis of
(-)-7-Deoxyloganin (12).
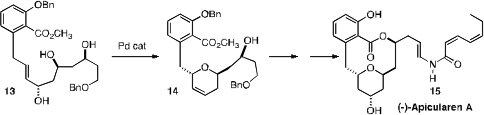
Jun’ichi Uenishi of Kyoto Pharmaceutical University showed
(Org. Lett. 2010, 12, 4160.
DOI: 10.1021/ol101753y)
that the Pd-mediated cyclization of 13 proceeded with high diastereocontrol.
Intramolecular esterification than led to (-)-Apicularen A (15).
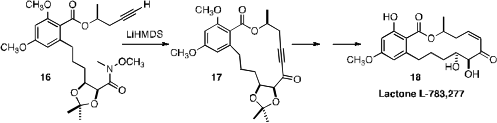
Martin G. Banwell of the Australian National University established
(Heterocycles 2010, 82, 313.
DOI: 10.3987/COM-10-S(E)67)
that LiHMDS was effective for the cyclization of the alkynyl Weinreb amide
16 to 17. Reduction and deprotection completed the
synthesis of the resorcylic lactone L-783, 277 (18).