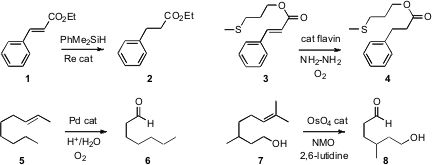
Ana C. Fernandes of the Instituto Superior Técnico, Lisboa, devised
(Tetrahedron Lett. 2010, 51, 1048.
DOI: 10.1016/j.tetlet.2009.12.061)
an effective Re catalyst for the solvent-free hydrogenation of an alkene 1.
Yasushi Imada and Takeshi Naota of Osaka University showed
(Org. Lett. 2010, 12, 32.
DOI: 10.1021/ol902393p)
that a flavin could catalyze the hydrogenation of an alkene 3. Note that
the thioether was stable under these conditions.
Huanfeng Jian of the South China University of Technology developed
(J. Org. Chem. 2010, 75, 2321.
DOI: 10.1021/jo100125q)
a Pd-based protocol for the
oxidative cleavage of an alkene 5. XPhos Pd G3 Order The cleavage
could be halted at the cis diol. K. C. Nicolaou of Scripps/La Jolla optimized
(Org. Lett. PMID:27102143 2010, 12, 1552.
DOI: 10.1021/ol100290a)
a complemetary cleavage of an alkene 7, again proceeding via the diol.
J. R. Falck of UT Southwestern established
(J. Formula of 1172057-73-6 Org. Chem. 2010,75, 1701.
DOI: 10.1021/jo902678p)
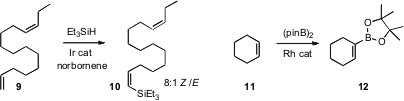
for the Heck-type oxidative silylation of an alkene 9 to
the Z-silane 10. Timothy F. Jamison of MIT effected
(Chem. Commun. 2010, 46, 907.
DOI: 10.1039/B921387B)
the borylation of an alkene 11. Kálmán Szabó of Stockholm University reported
(Angew. Chem. Int. Ed. 2010, 49, 4051.
DOI: 10.1002/anie.201000690)
a complementary approach for effecting the same
transformation. Cathleen M. Crudden of Queen’s University, Kingston, observed
(J. Am. Chem. Soc. 2010, 132, 131.
DOI: 10.1021/ja904142m)
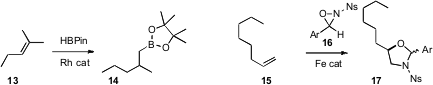
that Rh-catalyzed hydroboration of 13 delivered the borane 14.
Tehshik P. Yoon of the University of Wisconsin used
(J. Am. Chem. Soc. 2010, 132, 4570.
DOI: 10.1021/ja1013536)
Fe to catalyze the addition of an
oxaziridine 16 to an alkene 15.
Yasuhiro Shiraishi of Osaka University improved
(J. Org. Chem. 2010, 75, 1450.
DOI: 10.1021/jo902321f)
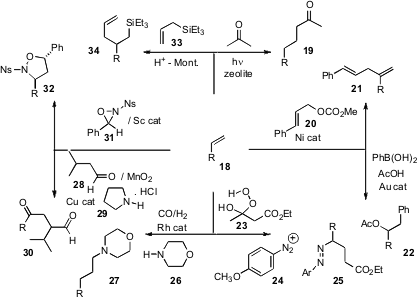
the photochemical addition of acetone to an alkene 18.
Chul-Ho Jun of Yonsei University described
(Tetrahedron Lett. 2010, 51, 160.
DOI: 10.1016/j.tetlet.2009.10.103)
a related procedure. Professor Jamison effected
(J. Am. Chem. Soc. 2010, 132, 6880.
DOI: 10.1021/ja101186p)
the branching homologation of an alkene to give 21.
F. Dean Toste of the University of California, Berkeley accomplished
(J. Am. Chem. Soc. 2010, 132, 8885.
DOI: 10.1021/ja1034123)
the oxidative homologation of an alkene to the ester 22. Markus R. Heinrich
of the Universität Erlangen-Nürnberg developed
(Tetrahedron Lett. 2010, 51, 1758.
DOI: 10.1016/j.tetlet.2010.01.098)
the tandem addition of the hydroperoxide 23 and a
diazonium salt 24, leading to 25.
Arno Behr of the Technische Universität Dortmund found
(Tetrahedron Lett. 2010, 51, 2438.
DOI: 10.1016/j.tetlet.2010.02.145)
aqueous conditions for aminomethylation, leading
to 27. Zhi-Zhen Huang of Nanjing University devised
(Chem. Commun. 2010, 46, 1947.
DOI: 10.1039/B921310D)
oxidative conditions for adding an aldehyde or ketone, to deliver the
1,4-dicarbonyl product 30. Professor Yoon established
(Angew. Chem. Int. Ed. 2010, 49, 930.
DOI: 10.1002/anie.200905801)
that that ScCl2(SbF6) could effect could effect the addition of
an oxaziridine 31, to give the homologated product 32.
Toshihide Baba of the Tokyo Institute of Technology developed
(Org. Lett. 2010, 12, 1508.
DOI: 10.1021/ol100228t)
a simple protocol for the addition of an allyl silane to give the branched silane 34.