Synthesis of (-)-Lasiol
Hisashi Yamamoto of the University of Chicago devised
(J. Am. Chem. Soc. 2010, 132, 7878.
DOI: 10.1021/ja100951u)
catalyst systems for the enantioselective
epoxidation of a Z-homoallylic
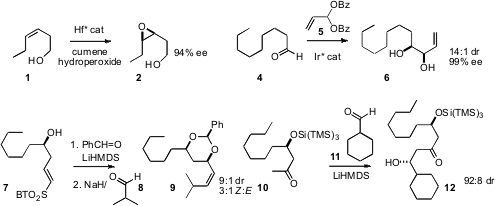
alcohol 1. 1310680-18-2 structure Michael J. 2-Octyldecanoic acid Price Krische of the University of Texas developed
(J. Am. Chem. PMID:24456950 Soc. 2010, 132, 1760.
DOI: 10.1021/ja9097675)
a catalyst system for the highly stereoselective addition of the vinyl acetal 5
to an aldehyde 4. Joëlle Prunet of the University of Glasgow showed
(Tetrahedron Lett. 2010, 51, 256.
DOI: 10.1016/j.tetlet.2009.10.139)
that the tandem cyclization/Julia olefination from 7 also proceeded
with high stereocontrol. Professor Yamamoto established
(J. Am. Chem. Soc. 2010, 132, 5354.
DOI: 10.1021/ja101076q)
that depending on conditions, the aldol condensation of 10 could
be directed selectively toward either diastereomer of the product 12.
James M. Takacs of the University of Nebraska effected
(J. Am. Chem. Soc. 2010, 132, 1740.
DOI: 10.1021/ja908257x)
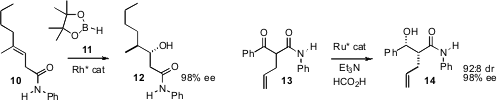
the enantioselective hydroboration of 10.
The other geometric isomer of 10 gave the alternative diastereomer of
12, also with high ee. John Limanto and Shane W. Krska of Merck Process optimized
(Org. Lett. 2010, 12, 512.
DOI: 10.1021/ol902715d)
the dynamic kinetic reduction of 13, to give 14 with
excellent diastereocontrol. Professor Krische extended
(J. Am. Chem. Soc. 2010, 132, 4562.
DOI: 10.1021/ja100949e) his reductive
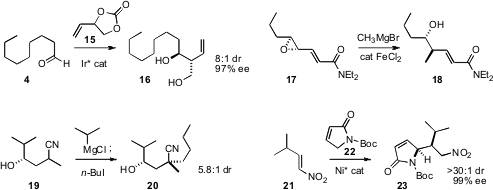
homologation to the (racemic) carbonate 15, delivering 16 with excellent
dr and ee. Hirokazu Urabe of the Tokyo Institute of Technology showed
(Org. Lett. 2010, 12, 1012.
DOI: 10.1021/ol100022w)
that a Grignard reagent under iron catalysis opened the epoxide 17,
readily available by Jørgensen-Cordova epoxidation followed by homologation, with
clean inversion and high regiocontrol. Fraser F. Fleming of Duquesne University developed
(Org. Lett. 2010, 12, 3030.
DOI: 10.1021/ol101030r)
a general route to quaternary alkylated centers, by alkylation of nitriles such
as 19. Shigeki Matsunaga and Masakatsu Shibasaki of the University of Tokyo devised
(J. Am. Chem. Soc. 2010, 132, 3666.
DOI: 10.1021/ja1002636)
a Ni catalyst for the stereoselective conjugate addition of the lactam 22 to a nitroalkene
21.
Aldehydes can also be added to nitroalkenes with high dr and ee, as
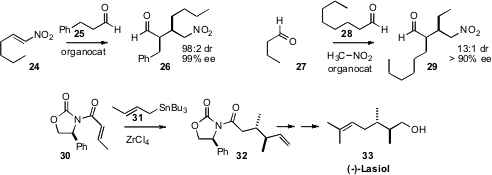
illustrated by the conversion of 24 to 26 reported
(J. Am. Chem. Soc. 2010, 132, 50.
DOI: 10.1021/ja9093583)
by Bukuo Ni of Texas A&M University-Commerce. Similar work by others
(J. Am. Chem. Soc. 2010, 132, 6,
DOI: 10.1021/ja9068112;
132, 4036,
DOI: 10.1021/ja909457b;
Org. Lett. 2010, 12, 1220,
DOI: 10.1021/ol9029758)
includes the remarkable triple combination of 27, 28, and
nitromethane described
(Angew. Chem. Int. Ed. 2010, 49, 2393.
DOI: 10.1002/anie.200902945)
by Jean M. J. Fréchet of the University of California, Berkeley. In the course of
a synthesis of (-)-Lasiol (33), Richard J. Mullins of Xavier University established
(Synlett 2010, 793.
DOI: 10.1055/s-0029-1219381)
a complementary strategy for installing adjacent alkylated stereogenic centers.