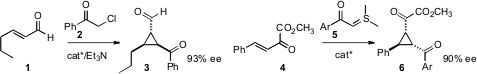B
Jinxing Ye of the East China University of Science and Technology used
(Tetrahedron Lett. PMID:23399686 Formula of 240401-09-6 2011, 52, 2715.
DOI: 10.1016/j.tetlet.2011.03.079)
the Hayashi catalyst to direct the
addition of 2 to 1, to give the
cyclopropane 3. Jia-Rong
Chen and Wen-Jing Xiao of Central China Normal University employed
(J. Org. Chem. 2011, 76, 281.
DOI: 10.1021/jo101699r)
a urea catalyst for the addition of 5 to 4.
Yasumasa Hamada of Chiba University devised
(Tetrahedron Lett. 823780-66-1 supplier 2011, 52, 987.
DOI: 10.1016/j.tetlet.2010.12.067)
a different urea catalyst for the addition of 7 to 8,
to control both the absolute and relative configuration of 9. Jiyong Hong
of Duke University showed
(Tetrahedron Lett. 2011, 52, 2468.
DOI: 10.1016/j.tetlet.2011.03.008)
that the imidazolium-mediated cyclization of 10 proceeded with high
diastereoselectivity, to give 11.
Yixin Lu of the National University of Singapore optimized
(J. Am. Chem. Soc. 2011, 133, 1726.
DOI: 10.1021/ja1106282)
a dipeptide-derived phosphine to catalyze the addition of 12 to 13.
Karl A. Scheidt of Northwestern University combined
(Angew. Chem. Int. Ed. 2011, 50, 1678.
DOI: 10.1002/anie.201005908)
a triazolium catalyst with super-stoichiometric Ti(O-iPr)4 to
effect the addition of 15 to 4, to give 16.
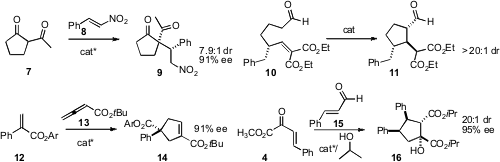
En route to Malyngamide C, Xiao-Ping Cao of Lanzhou University condensed
(J. Org. Chem. 2011, 76, 3946.
DOI: 10.1021/jo2003852)
the prochiral commercial monoketal 17 with nitrosobenzene, using proline
as organocatalyst, to prepare 18. Hong Wang of Miami University showed
(Angew. Chem. Int. Ed. 2011, 50, 3484.
DOI: 10.1002/anie.201100160)
that a lanthanide-complexed α-amino amide was effective for catalyzing
the addition of the prochiral 19 to 4, to give 20.
Alexandre Alexakis of the Universite de Geneve and John C. Stephens of the
National University of Ireland, Maynooth established
(Angew. Chem. Int. Ed. 2011, 50, 5095.
DOI: 10.1002/anie.201100804)
that the Hayashi catalyst was effective for
mediating the addition of 22 to 21, to give the diene 23.
Ying-Chun Chen of Sichuan University and Karl Anker Jørgensen of Aarhus
University used
(J. Am. Chem. Soc. 2011, 133, 5053.
DOI: 10.1021/ja1112194)
the same catalyst for the addition of 24 to 25. The Hayashi catalyst
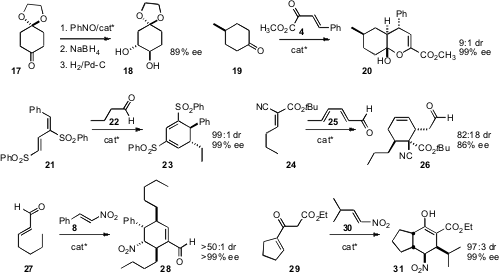
appeared again in the report
(Chem. Comm. 2011, 47, 3828.
DOI: 10.1039/C1CC10245A)
by Magnus Rueping of RWTH Aachen University of the addition of 27 to
28. Xiaohua Liu and Xiaoming Feng, also of Sichuan University, used
(Org. Lett. 2011, 13, 936.
DOI: 10.1021/ol1029832)
a Cinchona-derived catalyst to add 29 to 30.
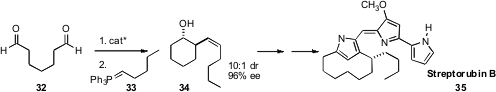
Regan J. Thomson, also of Northwestern University, took advantage
(J. Am. Chem. Soc. 2011, 133, 1799.
DOI: 10.1021/ja109165f)
of the proline-mediated intramolecular aldol condensation of 32 to prepare 34. Subsequent
oxy-Cope rearrangement established the ten-membered ring of Streptorubin B (35).