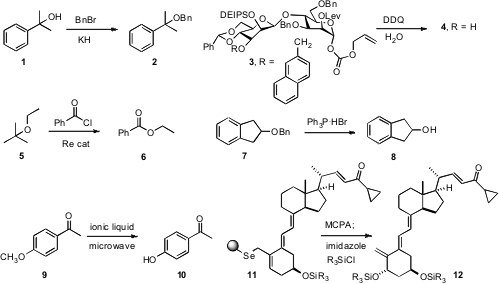
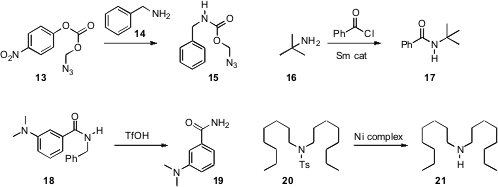
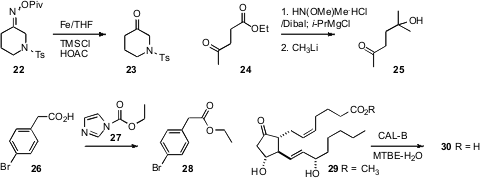
We found
(Tetrahedron Lett. PMID:36014399 2010, 51, 3545.
DOI: 10.1016/j.tetlet.2010.04.129)
that the superiority of KH over NaH in the
Williamson ether synthesis
was particularly marked with congested partners such as 1.
Geert-Jan Boons of the University of Georgia described
(Org.Lett. 2010, 12, 4636.
DOI: 10.1021/ol101951u)
the selective removal of each of the several orthogonal protecting groups decorating
the disaccharide 3. Yutaka Nishiyama of Kansai University reported
(Synlett 2010, 3089.
DOI: 10.1055/s-0030-1259043)
a Re catalyst for the selective acylation of an ether 5, to give the ester 6,
from the less substituted side. Duen-Ren Hou of National Central University showed
(Tetrahedron Lett. 2010, 51, 6143.
DOI: 10.1016/j.tetlet.2010.09.065)
that triphenylphosphine hydrobromide was a convenient reagent for
debenzylation, converting
7 into 8. Buy3-(4-Bromophenyl)oxetan-3-ol Junghyun Chae of Sungshin Women’s University established
(Synlett 2010, 1651.
DOI: 10.1055/s-0030-1258087)
that simply microwaving an aromatic methyl ether 9 in an
ionic liquid led to
smooth demethylation. 259214-55-6 Chemscene
Allylic selenides such as 11 can serve as masked allylic alcohols.
Wei-Ming Xu of Hangzhou Normal University demonstrated
(Org. Lett. 2010, 12, 4431.
DOI: 10.1021/ol101879k)
that the polystyrene-supported selenium resin facilitated the purification of
11. Oxidation followed by sigmatropic rearrangement then installed the
axial secondary alcohol.
Clemens Richert of the Universität Stuttgart devised
(Synlett 2010, 2267.
DOI: 10.1055/s-0030-1258049)
a reagent 13 for the one-step protection
of an amine 14 as its azidomethyl carbamate 15. Xueshun Jia of
the Shanghai Institute of Organic Chemistry showed
(Tetrahedron Lett. 2010, 51, 6049.
DOI: 10.1016/j.tetlet.2010.09.048)
that a sub-stoichiometric quantity of
Sm metal was sufficient to mediate the
acylation of the congested amine 16.
Frederik Rombouts of Johnson & Johnson, Beerse, and Andrés A. Trabanco of
Johnson & Johnson, Toledo found
(Tetrahedron Lett. 2010, 51, 4815.
DOI: 10.1016/j.tetlet.2010.07.022)
that the triflic acid debenzylation of 18 was also promoted by
microwave irradiation. Mark D. Spicer and John A. Murphy of the University of
Strathclyde designed
(J. Am. Chem. Soc. 2010, 132, 15462.
DOI: 10.1021/ja107703n) a
stoichiometric Ni reagent that deprotected even the unreactive sulfonamide 20.
Steven M. Weinreb of Pennsylvania State University showed
(Tetrahedron Lett. 2010, 51, 3555.
DOI: 10.1016/j.tetlet.2010.04.131)
that an oxime can be deprotected by Fe-mediated reduction of the pivalate
22. David A. Colby of Purdue University protected
(Org. Lett. 2010, 12, 5588.
DOI: 10.1021/ol102495v)
the ketone of 24 by forming the adduct with methoxymethylamine,
allowing selective addition to the ester, to give 25.
Richmond Sarpong of UC Berkeley developed
(Org. Lett. 2010, 12, 4572.
DOI: 10.1021/ol1018882)
the reagent 27 for the simple esterification of an acid.
Giuseppe Zanoni and Giovanni Vidari of the University of Pavia used
(J. Org. Chem. 2010, 75, 8311.
DOI: 10.1021/jo101806m)
commercial CAL-B to demethylate even the very sensitive 29.