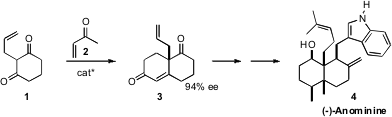
The Hajos-Parrish cyclization was a landmark in the asymmetric construction
of polycarbocyclic natural products. PMID:24220671 Impressive at the time, the
proline-mediated intramolecular
aldol condensation proceeded with an ee that was
low by modern standards. Ben Bradshaw and Josep Bonjoch of the Universitat de
Barcelona optimized this protocol, then used it to prepare
(J. Am. Chem. 1201644-34-9 web 1021-25-6 custom synthesis Soc. 2010, 132, 5966.
DOI: 10.1021/ja101994q)
the enone 3 en route to the Aspergillus
alkaloid (-)-Anominine (4).
The optimized catalyst for the enantioselective
Robinson annulation was the
amide 5. With 2.5 mol % of the catalyst, the reaction proceeded in 97% ee.
With only 1 mol % of catalyst, the reaction could be taken to 96% yield, while
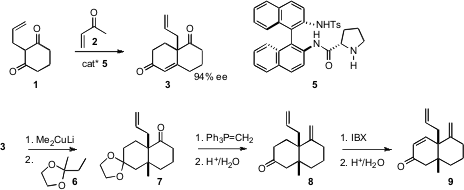
maintaining the ee at 94%. Conjugate addition proceeded across the open face of
3 to give, after selective protection, the monoketal 7. After
methylenation and deprotection,
oxidation with IBX delivered the enone 9.
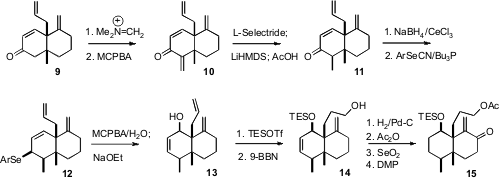
With the angular quaternary centers of the natural product in place, the
molecule became increasingly congested. Attempted direct alkylation of 9
led mainly to O-methylation. A solution to this problem was found in
condensation with the Eschenmoser salt, followed by N-oxide formation and
elimination to give the tetraene 10. Selective reduction by the Ganem
protocol followed by equilibration completed the net methylation.
Under anhydrous conditions, the oxide derived from the allylic selenide 12
did not rearrange. On the addition of water, the rearrangement proceeded
smoothly. Protection and hydroboration converted 13 into 14. The
bulk of the folded molecule protected the exo methylene of 14, so
hydrogenation followed by protection and oxidation delivered 15.
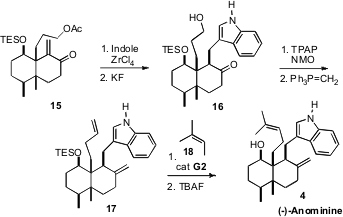
Conjugate addition of
indole to 15 set the stage for oxidation and
bis-methylenation to give 17. Selective Ru-mediated cross coupling with
18 followed by deprotection then completed the synthesis of (-)-Anominine
(4), which proved to be the enantiomer of the natural product.