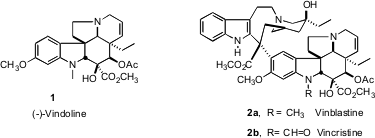
The periwinkle-derived alkaloids vinblastine (2a) and vincristine (2b)
are still mainstays of cancer chemotherapy. The more complex half of these
dimeric alkaloids, vindoline (1), presents a formidable challenge for
total synthesis. Building on his previous work
(Org. 2,5-Dimethoxyterephthalaldehyde Price Lett. 2005, 7, 4539.
DOI: 10.1021/ol051975x),
Dale L. Buy1885090-83-4 Boger of Scripps, La Jolla devised
(J. Am. Chem. Soc. PMID:24982871 2010, 132, 3685.
DOI: 10.1021/ja910695e)
a strikingly simple solution to this problem,
based on sequential cycloaddition.
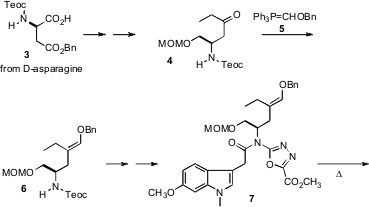
The starting point for the synthesis was the ester 3, derived from
D-asparagine. This was extended to 4, condensation of which with 5
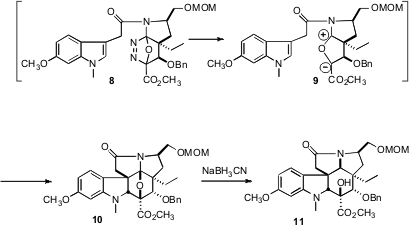
gave the enol ether 6. On heating, 7 cyclized to 8, which
lost N2 to give the zwitterion 9. Addition of the intermediate
9 to the indole then gave 10. In one reaction, the entire ring
system of vindoline, appropriately oxygenated, was assembled, with the original
stereogenic center from D-asparagine directing the relative and absolute
configuration of the final product.
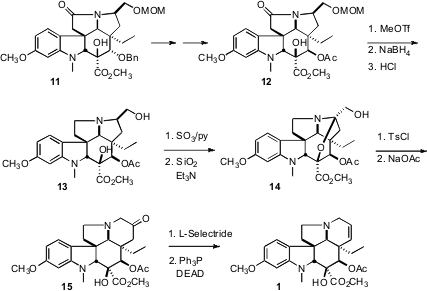
To complete the synthesis, the pendant carbon on 11 had to be
incorporated into the pentacyclic skeleton. After adjusting the relative
configuration of the secondary alcohol, the N was rendered nucleophilic by
reduction of the amide to the amine. Oxidation delivered 14, that on
activation as the tosylate smoothly rearranged to the ketone 15.
Reduction and regioselective dehydration then completed the synthesis of
vindoline (1).