Aspalathin (Minehan), (+)-Varitriol (Ghosh), Aspercyclide A (Spivey), Etnangien
(Menche)
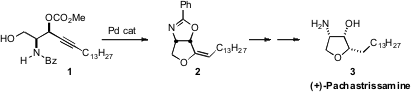
(+)-Pachastrissamine (3), also known as Jaspine B, induces apoptosis
in melanoma cells by a caspase-dependent pathway. Nobutaka Fujii and Hiroaki
Ohno of Kyoyo University developed
(J. Fmoc-α-Me-Gly(Pentynyl)-OH Chemical name Org. PMID:23600560 Price of 56946-65-7 Chem. 2010, 75, 3831.
DOI: 10.1021/jo100544v)
a practical route to 3 based on the Pd-mediated cyclization of 1
to 2.
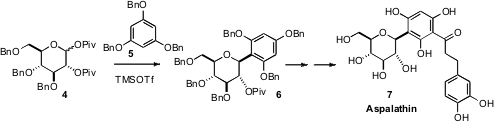
Thomas G. Minehan of California State University, Northridge optimized
(Org. Lett. 2010, 12, 1580.
DOI: 10.1021/ol100315g)
the condensation of 5 with the bis-pivalate 4. This opened a
general route to C-aryl glycosides, including Aspalathin (6).
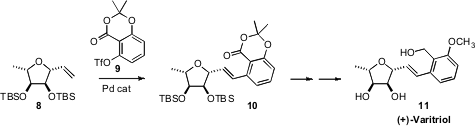
(+)-Varitriol (11) is vinylogously related to 7. A key step in
the synthesis of 11 reported
(J. Org. Chem. 2010, 75, 2107.
DOI: 10.1021/jo100001p)
by Subhash Ghosh of the Indian Institute of Chemical Technology was the
intermolecular Heck coupling of 8 with 9.
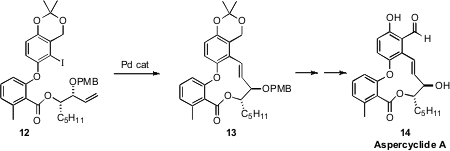
Aspercyclide A (14) and its more stable methyl ether are promising
lead compounds for the treatment of asthma. In the course of a synthesis of
14, Alan C. Spivey of Imperial College developed
(Chem. Commun. 2010, 1824.
DOI: 10.1039/B923528K)
the intramolecular Heck cyclization of 12 to 13.
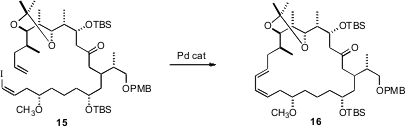
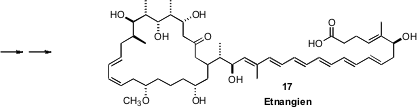
In a bold synthesis of Etnangien (17), Dirk Menche of
Ruprecht-Karls-Universität Heidelberg showed
(J. Org. Chem. 2010, 75, 2429.
DOI: 10.1021/ja9056163)
that the intramolecular Heck coupling of 15 to 16
proceeded efficiently. The substituents on 15 may be favoring
conformations that lead to cyclization.