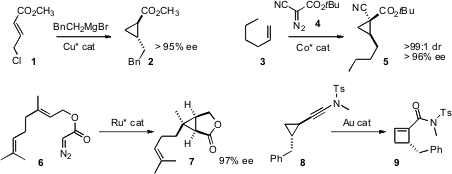
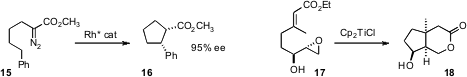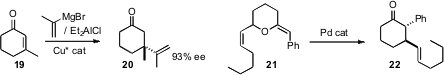Adriaan J. Minnaard and Ben L. Feringa of the University of Groningen devised
(J. Azido-PEG2-CH2COOH Price Am. Chem. Soc. 2010, 132, 14349.
DOI: 10.1021/ja105704m)
what promises to be a general strategy for the construction of enantiomerically-pure
cyclopropanes, based on
conjugate addition to acceptors such as 1. X. Peter
Zhang of the University of South Florida developed
(J. Am. Chem. Soc. 2010, 132, 12796.
DOI: 10.1021/ja1056246)
a Co catalyst for the enantioselective cyclopropanation of α-olefins
such as 3. Seiji Iwasa of Toyohashi University of Technology designed
(Angew. Chem. Int. Ed. 2010, 49, 8439.
DOI: 10.1002/anie.201002240)
a resin-bound Ru catalyst that could be used repeatedly for the enantioselective
cyclization of the ester 6. Rai-Shung Lin of National Tsing-Hua University showed
(Angew. PMID:24190482 5-Amino-3-methylindazole Data Sheet Chem. Int. Ed. 2010, 49, 9891.
DOI: 10.1002/anie.201004647)
that a gold catalyst could expand the alkyne 8 to the
cyclobutene 9.
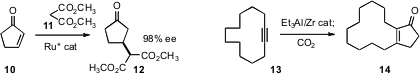
Takao Ikariya of the Tokyo Institute of Technology reported
(J. Am. Chem. Soc. 2010, 132, 16637.
DOI: 10.1021/ja107597w)
a detailed study of the enantioselective conjugate addition of malonate 11 to
cyclopentenone
10. Vladimir A. D’yakonov of the Russian Academy of Sciences, Ufa showed
(Tetrahedron Lett. 2010, 51, 5886.
DOI: 10.1016/j.tetlet.2010.08.120)
that a cyclic alkyne 13 could be annulated to the cyclopentenone
14. Shunichi Hashimoto of Hokkaido University also designed
(Angew. Chem. Int. Ed. 2010, 49, 6979.
DOI: 10.1002/anie.201003730)
a resin-bound Rh catalyst that could also be used repeatedly, for the enantioselective cyclization
of the ester 15. Tushar Kanti Chakraborty of the Central Drug Research Institute used
(Tetrahedron Lett. 2010, 51, 4425.
DOI: 10.1016/j.tetlet.2010.06.076)
Ti(III) to mediate the diastereoselective cyclization of 17 to 18.
Alexandre Alexakis of the University of Geneva extended
(Synlett 2010, 1694.
DOI: 10.1055/s-0029-1219958)
enantioselective conjugate addition of isopropenyl to the more difficult enone
19. Joseph P. A. Harrity of the University of Sheffield showed
(Org. Lett. 2010, 12, 4832.
DOI: 10.1021/ol102213s)
that Pd could catalyze the rearrangement of 21 to 22.
Strategies for the controlled construction of polycyclic ring systems are
also important. Günter Helmchen of the Universität Heidelberg showed
(J. Org. Chem. 2010, 75, 7917.
DOI: 10.1021/jo1014068)
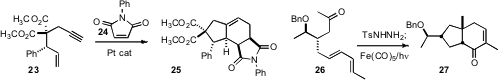
that 23 was efficiently cyclized to the diene with Pt catalyst. The reaction could
be carried out in the presence of the dienophile 24, to give 25 directly. We found
(J. Am. Chem. Soc. 2010, 132, 11179.
DOI: 10.1021/ja103551x)
that the cyclopropane formed from cyclization of the keto diene could be ring expanded
with CO to give the bicyclic enone 27.
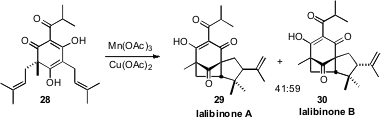
In pursuit of the tricyclic antibacterial ketones Ialibinone A (29) and Ialibinone B
(30), Nigel S. Simpkins of the University of Birmingham prepared
(Tetrahedron Lett. 2010, 51, 4823.
DOI: 10.1016/j.tetlet.2010.07.025)
the tetraketone 28. The radical from oxidation of 28 cyclized preferentially
onto one of the alkenes, to generate an intermediate radical that cyclized onto the other alkene.
The latter cyclization proceeded with modest diastereocontrol.