Asymmetric epoxidation of a prochiral alkene is an appealing process because two stereogenic centers are established in the course of the reaction. Often, the starting alkene is inexpensive. 1203499-17-5 Order There have been several interesting recent advances in the asymmetric nucleophilic epoxidation. 3-Bromo-4-methylaniline Chemical name
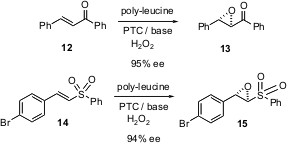
Keiji Maruoka of Kyoyo University reports (J. Am. Chem. PMID:23357584 Soc. 2004,126, 6844.DOI: 10.1021/ja048600b)the development of enantiomerically-pure quaternary ammonium salts (2) that catalyze the epoxidation of enones. The epoxidation of the t-butyl ketone 1 is particularly interesting, as Baeyer-Villiger oxidation would be expected to convert 3 into the ester4.
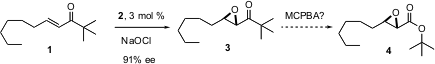
Masakatsu Shibasaki of the University of Tokyo reports (J. Am. Chem. Soc. 2004, 126, 7559.DOI: 10.1021/ja0485917)that use of a BINOL-derived catalyst withcumyl hydroperoxide enables the enantioselective epoxidation of unsaturatedN-acyl pyrroles such as 7. The pyrroles 7, prepared from the precursor aldehydes such as 5 with the reagent 6, can be used directly, without further purification.
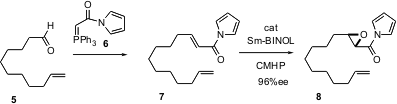
The product epoxy pyrroles such as 8 can be efficiently converted to the alcohol9, the homologated ester 10, and the homologated ketone 11.
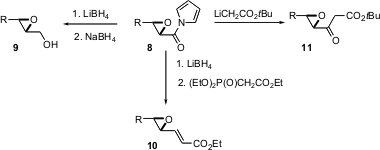
The Juliá-Colonna epoxidation uses poly-L-leucine and hydrogen peroxide to effect enantioselective epoxidation of chalcone derivatives such as12. In a pair of back-to-back papers (Tetrahedron Lett. 2004,45, 5065,DOI: 10.1016/j.tetlet.2004.04.188and 5069,DOI: 10.1016/j.tetlet.2004.04.189), H.-Christian Militzer of Bayer HealthCare AG, Wuppertal, reports a detailed optimization of this procedure. In the following paper (Tetrahedron Lett. 2004, 45, 5073.DOI: 10.1016/j.tetlet.2004.04.190), Stanley Roberts of the University of Liverpool reports the extension of this procedure to unsaturated sulfones such as14.