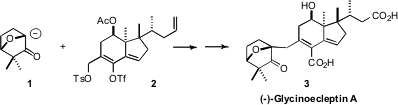
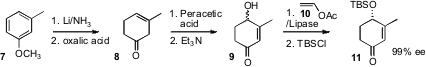(-)-Glycinoeclepin A (3) is effective at picogram/mL concentrations as
a hatch-stimulating agent for the soybean cyst nematode. Approaching the
synthesis of 3, Keiji Tanino of Hokkaido University envisioned
(Chem. Lett. 2010, 39, 835.
DOI: 10.1246/cl.2010.835)
the convergent coupling of the allylic
tosylate 2 with the bridgehead anion 1. Formula of 5458-56-0 4-Bromoisoquinolin-5-ol Chemical name The assembly of the
fragment 2 was particularly challenging, as the synthesis would require
the establishment not just of the two adjacent cyclic quaternary centers, but
also control of the relative configuration on the side chain. PMID:36628218
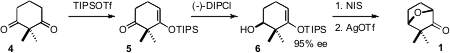
The preparation of 1 began with the prochiral diketone 3.
Enantioselective reduction of the mono enol ether 4 set the absolute
configuration of 5. Iodination followed by cyclization then completed the
assembly of 1.
The construction of the bicyclic tosylate 2 began with m-methyl
anisole (7). Following the
Rubottom procedure,
Birch reduction followed
by mild hydrolysis gave the ketone 8.
Epoxidation followed by
β-elimination delivered the racemic 9, which was exposed to lipase to give,
after seven days, the residual alcohol in 40% yield and high ee.
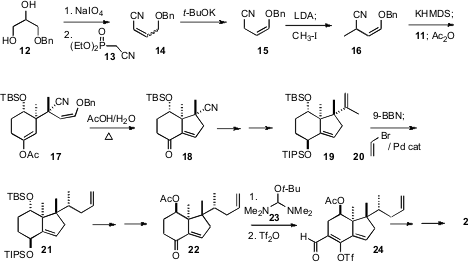
The side chain nitrile was prepared from the diol 12. Homologation gave the
nitrile 14, that was equilibrated to the more stable enol ether 15. The two
cyclic quaternary centers of 3 were set in a single step, by the conjugate
addition of the anion of 16 to the crystalline enone 11. Mild hydrolysis of
17 gave the keto aldehyde, that underwent aldol condensation to give the enone
18.
The hydroboration of 19 followed by
Suzuki coupling of the intermediate
organoborane with 20 delivered 21 with 94:6 relative
diastereocontrol. Formylation of the enone 22 followed by
triflation and reduction then led to 2.
Although the ketone 1 could be deprotonated with LDA, the only product
observed, even at -78°C, was the derived aldol dimer. The metalated
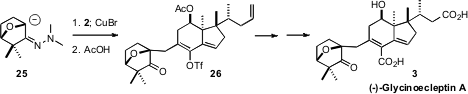
dimethylhydrazone 25, in contrast, coupled smoothly with 2 to give, after
hydrolyis, the desired adduct 26. Pd-mediated carboxylation of the enol triflate
followed by selective oxidative cleavage and hydrolysis then completed the
synthesis of (-)-Glycinoecleptin A (3).