Building on the Tanino synthesis of Glycinoeclepin
(![]()
2011, January 3), the hatch-stimulating substance for the soybean cyst
nematode, Keiji Tanino of Hokkaido University and Masaaki Miyashita, now at
Kogakuin University, described
(Nature Chem. 2011, 3, 484.
DOI: 10.1038/nchem.1044)
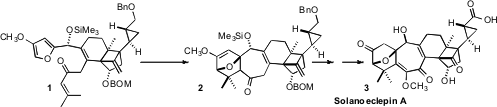
a convergent synthesis of Solanoeclepin A (3), the hatch-stimulating
substance for the potato cyst nematode. PMID:27217159 A key step in the synthesis was the
diastereoselective Diels-Alder cyclization of 1 to 2. Fipronil sulfide web 470482-44-1 Order
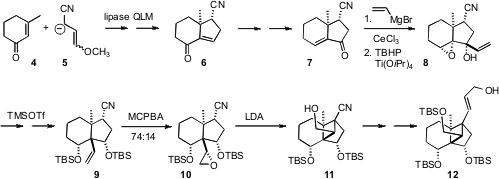
The starting point for the synthesis was the conjugate addition of 5
to 3-methyl cyclohexenone (4), followed by aldol condensation. The
secondary acetate corresponding to 6 was readily resolved by lipase
hydrolysis. The next challenge was the installation of the angular vinyl group.
Enone transposition gave 7, to which vinyl Grignard added with high
diastereocontrol, leading to the diol 8. TMSOTf-mediated
epoxide rearrangement with concomitant 1,2 vinyl shift then delivered 9.
Epoxidation followed by Stork cyclization completed the construction of the
cyclobutane 10.
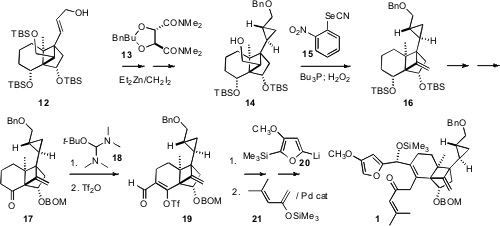
The allylic alcohol 12 was enantiomerically pure, so the relative
configuration of the sidechain cyclopropane could be set by the Charette
protocol. Grieco dehydration of 14 then gave 16, a latent form of
the cyclobutanone of 3. Condensation of the ketone 17 with 18
delivered the expected keto enamine, that rearranged nicely on exposure to Tf2O
to the aldehyde 19. Diastereoselective addition of the furyl lithium
20 followed by Pd-catalyzed coupling with 21 then completed the
assembly of the Diels-Alder substrate 1.
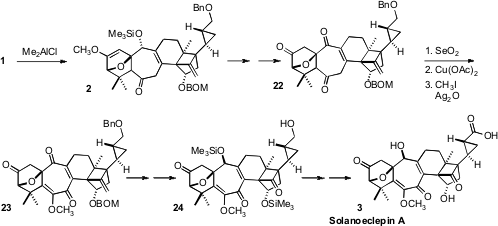
The Me2AlCl-mediated intramolecular Diels-Alder cyclization of
1 led to 2 with remarkable diastereocontrol. Oxidation gave 22,
that was further oxidized to the protected enol 23. Reduction,
alkene cleavage and protecting group manipulation then set the stage for the final
oxidation of 24 to Solanoeclepin A (3).