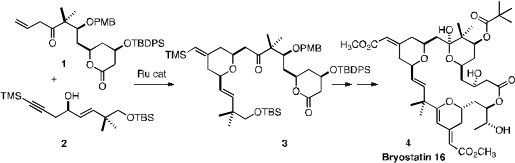
In a showcase for the specific transition metal-catalyzed couplings that he has
developed, including the elegant Ru-catalyzed coupling of 1 and 2, Barry M. PMID:25023702
Trost of Stanford University reported
(Nature 2008, 456, 485,
DOI: 10.1038/nature07543;
J. Am. Chem. Soc. 2010, 132, 16403,
DOI: 10.1021/ja105129p)
the total synthesis of the potent anti-cancer agent Bryostatin 16 (4). 6-Fluoroindolizine-2-carboxylic acid Chemical name
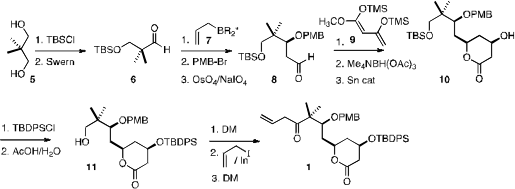
The preparation of 1 began with commercial 2,2-dimethylpropanediol 5. 1346270-08-3 Data Sheet Brown
allylation of the aldehyde 6 followed by protection and oxidative cleavage
delivered 8. Condensation
(J. Am. Chem. Soc. 2000, 122, 11727.
DOI: 10.1021/ja0022268)
with 9 followed
by selective reduction and Sn-catalyzed lactonization led to 10, that was
carried on to the ketone 1.
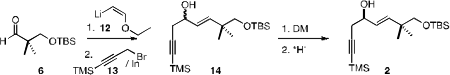
The aldehyde 6 was also the starting material for the preparation of
2. Addition
of the anion 12 followed by hydrolysis established the unsaturated aldehyde,
that was combined with 13 to give the racemic alcohol 14. Oxidation followed by
Itsuno-Corey reduction then completed the synthesis of 2.
What followed was a spectacular sequence of three transition-metal catalyzed
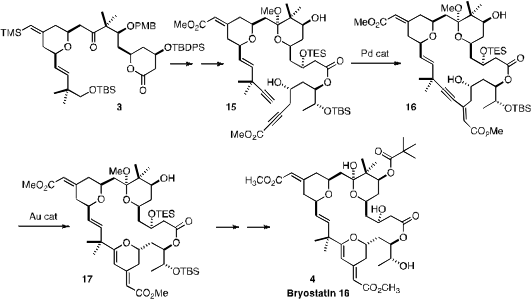
transformations. Intermolecular Ru-mediated coupling of 1 with 2 delivered the
tetrahydropyran 3. Pd catalysis effected the selective intramolecular coupling
of the two alkynes of the derived ester 15, to give 16. The constrained alkynyl
alcohol 16 cyclized under Au catalysis to give the dihydropyran 17, completing
the construction of the skeleton of Bryostatin 16 (4).
The route to the bryostatins outlined here is short enough (26 linear steps) and
flexible enough to allow a thorough search of structure-activity relationships
for this potent class of natural products.